当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient Synthesis of Quinazoline-2,4(1H,3H)-dione via Simultaneous Activated CO2 and 2-Aminobenzonitrile by 1-Methylhydantoin Anion-Functionalized Ionic Liquid through the Multiple-Site Cooperative Interactions
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2022-08-02 , DOI: 10.1021/acssuschemeng.2c03249 Tingting Chen 1 , Yunfei Zhang 1 , Yingjie Xu 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2022-08-02 , DOI: 10.1021/acssuschemeng.2c03249 Tingting Chen 1 , Yunfei Zhang 1 , Yingjie Xu 1
Affiliation
|
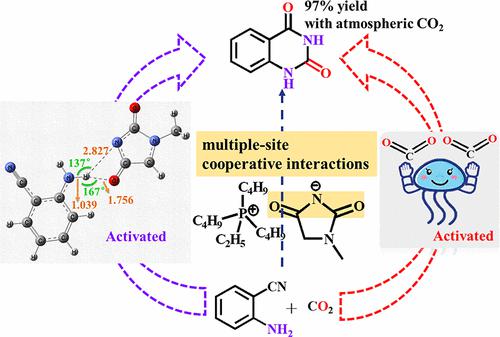
A novel basic anion-functionalized ionic liquid with a 1-methylhydantoin anion ([1-MHy]−) and [P4442]+ cation was synthesized for efficient CO2 capture and catalytic conversion of CO2 to quinazoline-2,4(1H,3H)-dione, and CO2 absorption and conversion mechanisms were investigated by NMR, FT-IR, and DFT calculations. CO2 absorption results show that the capture capacity of [P4442][1-MHy] at 303.15 K and 0.1 MPa is up to 1.58 molCO2/molIL, which is attributed to the multiple-site cooperative interactions between CO2 with N– and C═O of [1-MHy]− originated from the electron conjugation effect, resulting in a decrease in the CO2 absorption enthalpy (−51.4 kJ/mol) and an increase in the activation of CO2. Moreover, the high yield of quinazoline-2,4(1H,3H)-dione obtained by using [P4442][1-MHy] to catalyze 2-aminobenzonitrile with atmospheric-pressure CO2 was 97% and is solvent- and metal-free. The reaction mechanism indicates that [1-MHy]− with the multiple-site cooperative interactions can not only activate CO2 but also can activate 2-aminobenzonitrile by forming two types of hydrogen bonds with −NH2 (N–H···N– and N–H···O═C), thus ensuring the excellent catalytic performance of [P4442][1-MHy]. Furthermore, due to the similar basicity of [1-MHy]− to the quinazolide anion ([Quinazolide]−), it can effectively prevent the deprotonation of the acidic quinazoline-2,4-dione into [Quinazolide]−, thereby improving its yield. In addition, [P4442][1-MHy] has good recycling performance during CO2 capture and conversion. The physicochemical properties of [P4442][1-MHy] are also reported. The obtained results may provide a new strategy for converting CO2 into value-added chemicals under green conditions.
中文翻译:

1-甲基乙内酰脲阴离子功能化离子液体通过多位点协同作用同时活化CO2和2-氨基苯甲腈高效合成喹唑啉-2,4(1H,3H)-二酮
合成了一种具有 1-甲基乙内酰脲阴离子 ([1-MHy] - ) 和 [P 4442 ] +阳离子的新型碱性阴离子功能化离子液体,用于高效捕获 CO 2并将 CO 2催化转化为quinazoline-2,4(1通过NMR、FT-IR和DFT计算研究了H ,3 H )-二酮和CO 2的吸收和转化机理。CO 2吸收结果表明,[P 4442 ][1-MHy]在303.15 K和0.1 MPa下的捕获能力高达1.58 mol CO2 /mol IL,这归因于CO 2之间的多位点协同作用[1-MHy] - 的N -和C=O -源于电子共轭效应,导致CO 2吸收焓降低(-51.4 kJ/mol)和CO 2活化增加。此外,[P 4442 ][1-MHy]在常压CO 2下催化2-氨基苯甲腈得到喹唑啉-2,4(1 H ,3 H )-二酮的收率高达97%,是溶剂型并且不含金属。反应机理表明[1-MHy] -与多位点协同作用不仅可以激活CO 2还可以通过与-NH 2形成两种氢键(N-H···N-和N - H···O=C)活化2-氨基苄腈,从而保证了[P 4442 ]的优异催化性能[1-MHy]。此外,由于[1-MHy] -与喹唑啉阴离子([ Quinazolide] -)的碱性相似,它可以有效防止酸性喹唑啉-2,4-二酮去质子化为[Quinazolide] -,从而提高其屈服。此外,[P 4442 ][1-Mhy]在CO 2捕获和转化过程中具有良好的回收性能。[P 4442的理化性质][1-Mhy] 也有报道。所得结果可为在绿色条件下将CO 2转化为增值化学品提供新策略。
更新日期:2022-08-02
中文翻译:

1-甲基乙内酰脲阴离子功能化离子液体通过多位点协同作用同时活化CO2和2-氨基苯甲腈高效合成喹唑啉-2,4(1H,3H)-二酮
合成了一种具有 1-甲基乙内酰脲阴离子 ([1-MHy] - ) 和 [P 4442 ] +阳离子的新型碱性阴离子功能化离子液体,用于高效捕获 CO 2并将 CO 2催化转化为quinazoline-2,4(1通过NMR、FT-IR和DFT计算研究了H ,3 H )-二酮和CO 2的吸收和转化机理。CO 2吸收结果表明,[P 4442 ][1-MHy]在303.15 K和0.1 MPa下的捕获能力高达1.58 mol CO2 /mol IL,这归因于CO 2之间的多位点协同作用[1-MHy] - 的N -和C=O -源于电子共轭效应,导致CO 2吸收焓降低(-51.4 kJ/mol)和CO 2活化增加。此外,[P 4442 ][1-MHy]在常压CO 2下催化2-氨基苯甲腈得到喹唑啉-2,4(1 H ,3 H )-二酮的收率高达97%,是溶剂型并且不含金属。反应机理表明[1-MHy] -与多位点协同作用不仅可以激活CO 2还可以通过与-NH 2形成两种氢键(N-H···N-和N - H···O=C)活化2-氨基苄腈,从而保证了[P 4442 ]的优异催化性能[1-MHy]。此外,由于[1-MHy] -与喹唑啉阴离子([ Quinazolide] -)的碱性相似,它可以有效防止酸性喹唑啉-2,4-二酮去质子化为[Quinazolide] -,从而提高其屈服。此外,[P 4442 ][1-Mhy]在CO 2捕获和转化过程中具有良好的回收性能。[P 4442的理化性质][1-Mhy] 也有报道。所得结果可为在绿色条件下将CO 2转化为增值化学品提供新策略。






























 京公网安备 11010802027423号
京公网安备 11010802027423号