Bioactive Materials ( IF 18.0 ) Pub Date : 2022-08-02 , DOI: 10.1016/j.bioactmat.2022.07.016 Shuang Zeng 1 , Chen Chen 2 , Liuwei Zhang 1 , Xiaosheng Liu 1 , Ming Qian 1 , Hongyan Cui 1 , Jingyun Wang 1 , Qixian Chen 1 , Xiaojun Peng 2
|
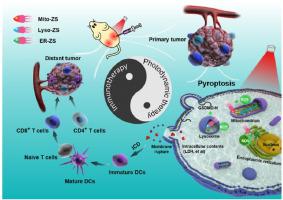
Pyroptosis, a unique lytic programmed cell death, inspired tempting implications as potent anti-tumor strategy in pertinent to its potentials in stimulating anti-tumor immunity for eradication of primary tumors and metastasis. Nonetheless, rare therapeutics have been reported to successfully stimulate pyroptosis. In view of the intimate participation of reactive oxygen species (ROS) in stimulating pyroptosis, we attempted to devise a spectrum of well-defined subcellular organelle (including mitochondria, lysosomes and endoplasmic reticulum)-targeting photosensitizers with the aim of precisely localizing ROS (produced from photosensitizers) at the subcellular compartments and explore their potentials in urging pyroptosis and immunogenic cell death (ICD). The subsequent investigations revealed varied degrees of pyroptosis upon photodynamic therapy (PDT) towards cancerous cells, as supported by not only observation of the distinctive morphological and mechanistic characteristics of pyroptosis, but for the first-time explicit validation from comprehensive RNA-Seq analysis. Furthermore, in vivo anti-tumor PDT could exert eradication of the primary tumors, more importantly suppressed the distant tumor and metastatic tumor growth through an abscopal effect, approving the acquirement of specific anti-tumor immunity as a consequence of pyroptosis. Hence, pyroptosis was concluded unprecedently by our proposed organelles-targeting PDT strategy and explicitly delineated with molecular insights into its occurrence and the consequent ICD.
中文翻译:

通过特定的细胞器靶向光动力疗法激活细胞焦亡以放大抗肿瘤免疫疗法的免疫原性细胞死亡
细胞焦亡是一种独特的裂解性程序性细胞死亡,作为有效的抗肿瘤策略激发了诱人的意义,与其刺激抗肿瘤免疫以根除原发性肿瘤和转移的潜力有关。尽管如此,据报道罕见的疗法可以成功刺激细胞焦亡。鉴于活性氧 (ROS) 密切参与刺激细胞焦亡,我们试图设计一系列定义明确的亚细胞器(包括线粒体、溶酶体和内质网)靶向光敏剂,目的是精确定位 ROS(产生的来自光敏剂)在亚细胞区室,并探索它们在促进细胞焦亡和免疫原性细胞死亡(ICD)方面的潜力。随后的研究揭示了对癌细胞进行光动力疗法 (PDT) 后不同程度的细胞焦亡,这不仅得到了对细胞焦亡独特形态学和机制特征的观察的支持,而且得到了综合 RNA-Seq 分析的首次明确验证。此外,体内抗肿瘤PDT可发挥根除原发性肿瘤,更重要的是通过远隔效应抑制远处肿瘤和转移性肿瘤的生长,批准细胞焦亡获得特异性抗肿瘤免疫。因此,我们提出的细胞器靶向 PDT 策略前所未有地得出了细胞焦亡的结论,并通过对其发生和随之而来的 ICD 的分子洞察明确描述。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号