当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Carbene Transfer from a Pyridine Dipyrrolide Iron–Carbene Complex: Reversible Migration of a Diphenylcarbene Ligand into an Iron–Nitrogen Bond
Organometallics ( IF 2.5 ) Pub Date : 2022-08-01 , DOI: 10.1021/acs.organomet.2c00260 Brett M. Hakey 1 , Dylan C. Leary 1 , Jordan C. Martinez 1 , Jonathan M. Darmon 2 , Novruz G. Akhmedov 1 , Jeffrey L. Petersen 1 , Carsten Milsmann 1
Organometallics ( IF 2.5 ) Pub Date : 2022-08-01 , DOI: 10.1021/acs.organomet.2c00260 Brett M. Hakey 1 , Dylan C. Leary 1 , Jordan C. Martinez 1 , Jonathan M. Darmon 2 , Novruz G. Akhmedov 1 , Jeffrey L. Petersen 1 , Carsten Milsmann 1
Affiliation
|
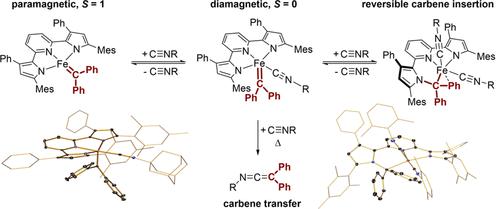
The reaction between the square-planar, paramagnetic iron–carbene complex (MesPDPPh)Fe(CPh2) (where [MesPDPPh]2– is the doubly deprotonated form of 2,6-bis(5-(2,4,6-trimethylphenyl)-3-phenyl-1H-pyrrol-2-yl)pyridine) and two equivalents of carbon monoxide or 2,6-dimethylphenylisocyanide, CNdmp, resulted in carbene insertion into a Fe–Npyrrole bond of the MesPDPPh ligand. Crystallographic studies established complete scission of the Fe–Npyrrole bond in the resulting low-spin FeII complexes. Experiments using 1-adamantylisocyanide, CN1Ad, demonstrated that binding of two exogenous ligands was required to promote carbene migration and allowed the isolation of diamagnetic (MesPDPPh)Fe(CPh2)(CN1Ad). Computational studies by density functional theory (DFT) and complete-active-space self-consistent field (CASSCF) calculations support that (MesPDPPh)Fe(CPh2)(CN1Ad) possesses a similar electronic structure to porphyrin iron carbenes, best described as containing a neutral Fischer-type carbene coordinated to a low-spin FeII center. DFT calculations also suggested that carbene migration is triggered by an increase in carbene electrophilicity upon binding of a second isocyanide ligand. Thermolysis of the carbene insertion product obtained following the addition of CNdmp to (MesPDPPh)Fe(CPh2) revealed that the insertion is fully reversible and provided the ketenimine N-(2,2-diphenylethenylidene)-2,6-dimethylbenzenamine as the major product.
中文翻译:

从吡啶二吡咯内酯铁-卡宾配合物中的卡宾转移:二苯基卡宾配体可逆迁移到铁-氮键中
方形平面顺磁性铁-卡宾配合物 ( Mes PDP Ph )Fe(CPh 2 ) 之间的反应(其中 [ Mes PDP Ph ] 2–是 2,6-bis(5-(2,4 ,6-三甲基苯基)-3-苯基-1 H-吡咯-2-基)吡啶)和两个当量的一氧化碳或 2,6-二甲基苯基异氰化物 CNdmp 导致卡宾插入Mes的 Fe-N吡咯键PDP Ph配体。晶体学研究确定了在所得低自旋 Fe II中 Fe-N吡咯键的完全断裂配合物。使用 1-金刚烷基异氰化物 CN 1 Ad 的实验表明,需要结合两个外源配体来促进卡宾迁移并允许分离抗磁性 ( Mes PDP Ph )Fe(CPh 2 )(CN 1 Ad)。通过密度泛函理论 (DFT) 和完全活性空间自洽场 (CASSCF) 计算的计算研究支持 ( Mes PDP Ph )Fe(CPh 2 )(CN 1 Ad) 具有与卟啉铁卡宾类似的电子结构,最好描述为含有与低自旋 Fe II配位的中性 Fischer 型卡宾中央。DFT 计算还表明,卡宾迁移是由与第二个异氰化物配体结合后卡宾亲电性的增加引发的。将 CNdmp 添加到 ( Mes PDP Ph )Fe(CPh 2 ) 后获得的卡宾插入产物的热解表明,插入是完全可逆的,并提供了酮亚胺N -(2,2-二苯基亚乙基)-2,6-二甲基苯胺主要产品。
更新日期:2022-08-01
中文翻译:

从吡啶二吡咯内酯铁-卡宾配合物中的卡宾转移:二苯基卡宾配体可逆迁移到铁-氮键中
方形平面顺磁性铁-卡宾配合物 ( Mes PDP Ph )Fe(CPh 2 ) 之间的反应(其中 [ Mes PDP Ph ] 2–是 2,6-bis(5-(2,4 ,6-三甲基苯基)-3-苯基-1 H-吡咯-2-基)吡啶)和两个当量的一氧化碳或 2,6-二甲基苯基异氰化物 CNdmp 导致卡宾插入Mes的 Fe-N吡咯键PDP Ph配体。晶体学研究确定了在所得低自旋 Fe II中 Fe-N吡咯键的完全断裂配合物。使用 1-金刚烷基异氰化物 CN 1 Ad 的实验表明,需要结合两个外源配体来促进卡宾迁移并允许分离抗磁性 ( Mes PDP Ph )Fe(CPh 2 )(CN 1 Ad)。通过密度泛函理论 (DFT) 和完全活性空间自洽场 (CASSCF) 计算的计算研究支持 ( Mes PDP Ph )Fe(CPh 2 )(CN 1 Ad) 具有与卟啉铁卡宾类似的电子结构,最好描述为含有与低自旋 Fe II配位的中性 Fischer 型卡宾中央。DFT 计算还表明,卡宾迁移是由与第二个异氰化物配体结合后卡宾亲电性的增加引发的。将 CNdmp 添加到 ( Mes PDP Ph )Fe(CPh 2 ) 后获得的卡宾插入产物的热解表明,插入是完全可逆的,并提供了酮亚胺N -(2,2-二苯基亚乙基)-2,6-二甲基苯胺主要产品。






























 京公网安备 11010802027423号
京公网安备 11010802027423号