当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Separation of Styrene and Ethylbenzene on Metal−Organic Frameworks: Analogous Structures with Different Adsorption Mechanisms
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2010-11-03 , DOI: 10.1021/ja106142x
Michael Maes 1 , Frederik Vermoortele 1 , Luc Alaerts 1 , Sarah Couck 1 , Christine E. A. Kirschhock 1 , Joeri F. M. Denayer 1 , Dirk E. De Vos 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2010-11-03 , DOI: 10.1021/ja106142x
Michael Maes 1 , Frederik Vermoortele 1 , Luc Alaerts 1 , Sarah Couck 1 , Christine E. A. Kirschhock 1 , Joeri F. M. Denayer 1 , Dirk E. De Vos 1
Affiliation
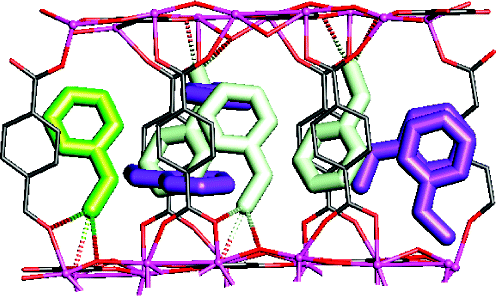
|
The metal-organic frameworks MIL-47 (V(IV)O{O(2)C-C(6)H(4)-CO(2)}) and MIL-53(Al) (Al(III)(OH)·{O(2)C-C(6)H(4)-CO(2)}) are capable of separating ethylbenzene and styrene. Both materials adsorb up to 20-24 wt % of both compounds. Despite the fact that they have identical building schemes, the reason for preferential adsorption of styrene compared to ethylbenzene is very different for the two frameworks. For MIL-47, diffraction experiments reveal that styrene is packed inside the pores in a unique, pairwise fashion, resulting in separation factors as high as 4 in favor of styrene. These separation factors are independent of the total amount of adsorbate offered. This is due to co-adsorption of ethylbenzene in the space left available between the packed styrene pairs. The separation is of a non-enthalpic nature. On MIL-53, the origin of the preferential adsorption of styrene is related to differences in enthalpy of adsorption, which are based on different degrees of framework relaxation. The proposed adsorption mechanisms are in line with the influence of temperature on the separation factors derived from pulse chromatography: separation factors are independent of temperature for MIL-47 but vary with temperature for MIL-53. Finally, MIL-53 is also capable of removing typical impurities like o-xylene or toluene from styrene-ethylbenzene mixtures.
中文翻译:

在金属-有机骨架上分离苯乙烯和乙苯:具有不同吸附机制的类似结构
金属有机骨架 MIL-47 (V(IV)O{O(2)CC(6)H(4)-CO(2)}) 和 MIL-53(Al) (Al(III)(OH)· {O(2)CC(6)H(4)-CO(2)}) 能够分离乙苯和苯乙烯。两种材料最多可吸附 20-24 重量%的两种化合物。尽管它们具有相同的构建方案,但与乙苯相比,这两种框架优先吸附苯乙烯的原因非常不同。对于 MIL-47,衍射实验表明苯乙烯以独特的成对方式填充在孔内,导致分离因子高达 4,有利于苯乙烯。这些分离因素与所提供的吸附物总量无关。这是由于在填充的苯乙烯对之间留下的可用空间中乙苯共吸附。分离具有非焓性质。在 MIL-53 上,苯乙烯优先吸附的起源与吸附焓的差异有关,这是基于不同程度的骨架松弛。所提出的吸附机制与温度对脉冲色谱分离因子的影响一致:MIL-47 的分离因子与温度无关,但 MIL-53 的分离因子随温度变化。最后,MIL-53 还能够从苯乙烯-乙苯混合物中去除典型的杂质,如邻二甲苯或甲苯。MIL-47 的分离系数与温度无关,但 MIL-53 的分离系数随温度变化。最后,MIL-53 还能够从苯乙烯-乙苯混合物中去除典型的杂质,如邻二甲苯或甲苯。MIL-47 的分离系数与温度无关,但 MIL-53 的分离系数随温度变化。最后,MIL-53 还能够从苯乙烯-乙苯混合物中去除典型的杂质,如邻二甲苯或甲苯。
更新日期:2010-11-03
中文翻译:

在金属-有机骨架上分离苯乙烯和乙苯:具有不同吸附机制的类似结构
金属有机骨架 MIL-47 (V(IV)O{O(2)CC(6)H(4)-CO(2)}) 和 MIL-53(Al) (Al(III)(OH)· {O(2)CC(6)H(4)-CO(2)}) 能够分离乙苯和苯乙烯。两种材料最多可吸附 20-24 重量%的两种化合物。尽管它们具有相同的构建方案,但与乙苯相比,这两种框架优先吸附苯乙烯的原因非常不同。对于 MIL-47,衍射实验表明苯乙烯以独特的成对方式填充在孔内,导致分离因子高达 4,有利于苯乙烯。这些分离因素与所提供的吸附物总量无关。这是由于在填充的苯乙烯对之间留下的可用空间中乙苯共吸附。分离具有非焓性质。在 MIL-53 上,苯乙烯优先吸附的起源与吸附焓的差异有关,这是基于不同程度的骨架松弛。所提出的吸附机制与温度对脉冲色谱分离因子的影响一致:MIL-47 的分离因子与温度无关,但 MIL-53 的分离因子随温度变化。最后,MIL-53 还能够从苯乙烯-乙苯混合物中去除典型的杂质,如邻二甲苯或甲苯。MIL-47 的分离系数与温度无关,但 MIL-53 的分离系数随温度变化。最后,MIL-53 还能够从苯乙烯-乙苯混合物中去除典型的杂质,如邻二甲苯或甲苯。MIL-47 的分离系数与温度无关,但 MIL-53 的分离系数随温度变化。最后,MIL-53 还能够从苯乙烯-乙苯混合物中去除典型的杂质,如邻二甲苯或甲苯。



































 京公网安备 11010802027423号
京公网安备 11010802027423号