当前位置:
X-MOL 学术
›
ACS ES&T Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synergistic Degradation of 2,4,4′-Trihydroxybenzophenone Using Carbon Quantum Dots, Ferrate, and Visible Light Irradiation: Insights into Electron Generation/Consumption Mechanism
ACS ES&T Engineering ( IF 7.4 ) Pub Date : 2022-07-28 , DOI: 10.1021/acsestengg.2c00118 Afzal Ahmed Dar 1 , Muhammad Usman 2 , Wei Zhang 1 , Qiuhui Zhu 1 , Bao Pan 3 , Atif Sial 1 , Chuanyi Wang 1
ACS ES&T Engineering ( IF 7.4 ) Pub Date : 2022-07-28 , DOI: 10.1021/acsestengg.2c00118 Afzal Ahmed Dar 1 , Muhammad Usman 2 , Wei Zhang 1 , Qiuhui Zhu 1 , Bao Pan 3 , Atif Sial 1 , Chuanyi Wang 1
Affiliation
|
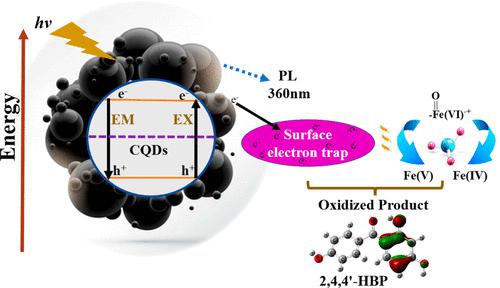
In this study, we reported a unique single-step synthesis of photocatalytic carbon quantum dots (CQDs) with high electron density, efficient aggregation-induced emission, and improved surface chemistry properties. As-synthesized CQDs were applied with ferrate (a reactive and green oxidant) to synergistically degrade 2,4,4′-trihydroxybenzophenone (2,4,4′-HBP). The restriction of molecular rotation, vibration in the crystal structure, modification of the surface chemistry, and the aggregation/quenching of CQDs were highlighted by comprehensive characterization. Newly synthesized CQDs’ generated electrons were consumed by ferrate, and this mechanism was highlighted by photoluminescence analyses. It was found that the photoluminescence intensity of CQDs was reduced by more than 96% when ferrate was introduced into the CQDs reaction system. The reaction kinetics of 2,4,4′-HBP degradation was inspected at different pH values (7.0, 8.0 and 9.0) using ferrate in a single dose and multiple-dose (sequential) additions. The CQDs + ferrate + visible light (Vis) was proven to be the best reaction system with a 99.9% degradation efficiency, as compared to CQDs alone (63%) and ferrate single dose (85%) and multiple-dose (96%) methods. In addition, 10 reaction intermediates were identified, implying that C–C cleavage, hydroxylation, self, cross, and end linkage were involved in the potential reaction mechanism. Besides, hydroxyl radicals were found to be the primary reactive species in electron paramagnetic resonance (EPR) analyses. The computational analyses including density functional theory and molecular orbital distribution elucidated the reaction sites of CQDs and 2,4,4′-HBP. These findings have important implications in understanding electron generation and consumption mechanisms with the synergetic contribution of CQDs and ferrate to degrade organic pollutants such as 2,4,4′-HBP.
中文翻译:

使用碳量子点、高铁酸盐和可见光照射协同降解 2,4,4'-三羟基二苯甲酮:深入了解电子产生/消耗机制
在这项研究中,我们报道了一种独特的单步合成光催化碳量子点 (CQD) 的方法,该方法具有高电子密度、有效的聚集诱导发射和改进的表面化学性质。将合成的 CQD 与高铁酸盐(一种活性和绿色氧化剂)一起应用以协同降解 2,4,4'-三羟基二苯甲酮(2,4,4'-HBP)。通过综合表征突出了分子旋转的限制、晶体结构中的振动、表面化学的改性以及 CQD 的聚集/猝灭。新合成的 CQD 产生的电子被高铁酸盐消耗,光致发光分析强调了这种机制。结果表明,将高铁酸盐引入CQDs反应体系后,CQDs的光致发光强度降低了96%以上。在不同 pH 值(7.0、8.0 和 9.0)下使用单剂量和多剂量(连续)添加的高铁酸盐检查 2,4,4'-HBP 降解的反应动力学。CQDs + 高铁酸盐 + 可见光 (Vis) 被证明是最好的反应系统,与单独的 CQDs (63%) 和高铁酸盐单剂量 (85%) 和多剂量 (96%) 相比,降解效率为 99.9%方法。此外,鉴定出 10 个反应中间体,表明 C-C 裂解、羟基化、自身、交叉和末端连接参与了潜在的反应机理。此外,发现羟基自由基是电子顺磁共振(EPR)分析中的主要活性物质。包括密度泛函理论和分子轨道分布在内的计算分析阐明了CQDs和2,4,4'-HBP的反应位点。
更新日期:2022-07-28
中文翻译:

使用碳量子点、高铁酸盐和可见光照射协同降解 2,4,4'-三羟基二苯甲酮:深入了解电子产生/消耗机制
在这项研究中,我们报道了一种独特的单步合成光催化碳量子点 (CQD) 的方法,该方法具有高电子密度、有效的聚集诱导发射和改进的表面化学性质。将合成的 CQD 与高铁酸盐(一种活性和绿色氧化剂)一起应用以协同降解 2,4,4'-三羟基二苯甲酮(2,4,4'-HBP)。通过综合表征突出了分子旋转的限制、晶体结构中的振动、表面化学的改性以及 CQD 的聚集/猝灭。新合成的 CQD 产生的电子被高铁酸盐消耗,光致发光分析强调了这种机制。结果表明,将高铁酸盐引入CQDs反应体系后,CQDs的光致发光强度降低了96%以上。在不同 pH 值(7.0、8.0 和 9.0)下使用单剂量和多剂量(连续)添加的高铁酸盐检查 2,4,4'-HBP 降解的反应动力学。CQDs + 高铁酸盐 + 可见光 (Vis) 被证明是最好的反应系统,与单独的 CQDs (63%) 和高铁酸盐单剂量 (85%) 和多剂量 (96%) 相比,降解效率为 99.9%方法。此外,鉴定出 10 个反应中间体,表明 C-C 裂解、羟基化、自身、交叉和末端连接参与了潜在的反应机理。此外,发现羟基自由基是电子顺磁共振(EPR)分析中的主要活性物质。包括密度泛函理论和分子轨道分布在内的计算分析阐明了CQDs和2,4,4'-HBP的反应位点。






























 京公网安备 11010802027423号
京公网安备 11010802027423号