当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
2-Oxa-5-azabicyclo[2.2.1]heptane as a Platform for Functional Diversity: Synthesis of Backbone-Constrained γ-Amino Acid Analogues
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-07-28 , DOI: 10.1021/acs.joc.2c01338 Jean-Baptiste Garsi 1 , Solène Guggari 1 , Thomas Deis 1 , Myles Ma 1 , Sofiane Hocine 1 , Stephen Hanessian 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-07-28 , DOI: 10.1021/acs.joc.2c01338 Jean-Baptiste Garsi 1 , Solène Guggari 1 , Thomas Deis 1 , Myles Ma 1 , Sofiane Hocine 1 , Stephen Hanessian 1
Affiliation
|
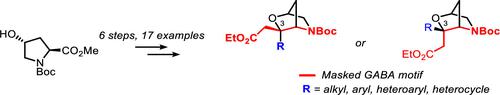
We communicate a versatile synthetic approach to C-3 disubstituted 2-oxa-5-azabicyclo[2.2.1]heptanes as carbon-atom bridged morpholines, starting with 4R-hydroxy-l-proline as a chiron. Attaching an acetic acid moiety on the C-3 carbon of the 2-oxa-5-azabicyclo[2.2.1]heptane core reveals the framework of an embedded γ-amino butyric acid (GABA). Variations in the nature of the substituent on the tertiary C-3 atom with different alkyls or aryls led to backbone-constrained analogues of the U.S. Food and Drug Administration-approved drugs baclofen and pregabalin.
中文翻译:

2-Oxa-5-azabicyclo[2.2.1]heptane 作为功能多样性的平台:骨架约束 γ-氨基酸类似物的合成
我们将 C-3 二取代的 2-oxa-5-azabicyclo[2.2.1]heptanes 作为碳原子桥联吗啉,以 4 R -羟基-l-脯氨酸作为 chiron 开始,传达了一种通用的合成方法。在 2-oxa-5-azabicyclo[2.2.1]庚烷核心的 C-3 碳上附加一个乙酸部分揭示了嵌入的 γ-氨基丁酸 (GABA) 的框架。具有不同烷基或芳基的叔 C-3 原子上的取代基性质的变化导致了美国食品和药物管理局批准的药物巴氯芬和普瑞巴林的骨架受限类似物。
更新日期:2022-07-28
中文翻译:

2-Oxa-5-azabicyclo[2.2.1]heptane 作为功能多样性的平台:骨架约束 γ-氨基酸类似物的合成
我们将 C-3 二取代的 2-oxa-5-azabicyclo[2.2.1]heptanes 作为碳原子桥联吗啉,以 4 R -羟基-l-脯氨酸作为 chiron 开始,传达了一种通用的合成方法。在 2-oxa-5-azabicyclo[2.2.1]庚烷核心的 C-3 碳上附加一个乙酸部分揭示了嵌入的 γ-氨基丁酸 (GABA) 的框架。具有不同烷基或芳基的叔 C-3 原子上的取代基性质的变化导致了美国食品和药物管理局批准的药物巴氯芬和普瑞巴林的骨架受限类似物。






























 京公网安备 11010802027423号
京公网安备 11010802027423号