Journal of Organometallic Chemistry ( IF 2.1 ) Pub Date : 2022-07-27 , DOI: 10.1016/j.jorganchem.2022.122468 Zekeriya Biyiklioglu , Turgut Keleş , Huseyin Sahin
|
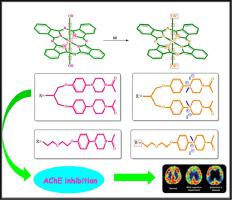
In this paper, axial 1,3-bis[4-(4-acetylpiperazin-1-yl)phenoxy]propanoxy and {2-[4-(4-acetylpiperazin-1-yl)phenoxy]ethoxy}ethoxy groups substituted silicon(IV) phthalocyanines (PP-D-Si, PP-OH2-Si) and their quaternized derivatives (PP-D-SiQ, PP-OH2-SiQ) were synthesized and characterized. The acetylcholinesterase inhibition values of 1,3-bis[4-(4-acetylpiperazin-1-yl)phenoxy]propanoxy and {2-[4-(4-acetylpiperazin-1-yl)phenoxy]ethoxy}ethoxy groups substituted silicon(IV) phthalocyanines (PP-D-Si, PP-OH2-Si) and their quaternized derivatives (PP-D-SiQ, PP-OH2-SiQ) were measured by IC50 that reduces enzyme activity to 50% refers to the concentration of inhibitor. The synthesis compounds were classified as silicon and their quaternized derivatives and tagged as PP-D-Si, PP-OH2-Si, PP-D-SiQ and PP-OH2-SiQ. Except for the result of PP-D-SiQ was 1.586 ± 0.129 µM, the results were expressed as mM ranged between 0.553 and 3.626 mM.
中文翻译:

轴向二取代硅酞菁及其季铵化衍生物的合成及乙酰胆碱酯酶抑制特性
本文采用轴向1,3-双[4-(4-乙酰哌嗪-1-基)苯氧基]丙氧基和{2-[4-(4-乙酰哌嗪-1-基)苯氧基]乙氧基}乙氧基取代硅( IV)合成并表征了酞菁( PP-D-Si、PP-OH2-Si )及其季铵化衍生物( PP-D-SiQ、PP-OH2-SiQ )。1,3-双[4-(4-乙酰哌嗪-1-基)苯氧基]丙氧基和{2-[4-(4-乙酰哌嗪-1-基)苯氧基]乙氧基}乙氧基取代硅( IV) 酞菁(PP-D-Si、PP-OH2-Si)及其季铵化衍生物(PP-D-SiQ、PP-OH2-SiQ)通过IC 50测量将酶活性降低到 50% 是指抑制剂的浓度。合成化合物分类为硅及其季铵化衍生物,并标记为PP-D-Si、PP-OH2-Si、PP-D-SiQ和PP-OH2-SiQ。除了PP-D-SiQ的结果为 1.586 ± 0.129 µM 外,结果以 mM 表示,范围在 0.553 和 3.626 mM 之间。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号