当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Products and Mechanisms of Secondary Organic Aerosol Formation from the NO3 Radical-Initiated Oxidation of Cyclic and Acyclic Monoterpenes
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2022-07-26 , DOI: 10.1021/acsearthspacechem.2c00130 Marla P. DeVault 1, 2 , Anna C. Ziola 1, 2 , Paul J. Ziemann 1, 2
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2022-07-26 , DOI: 10.1021/acsearthspacechem.2c00130 Marla P. DeVault 1, 2 , Anna C. Ziola 1, 2 , Paul J. Ziemann 1, 2
Affiliation
|
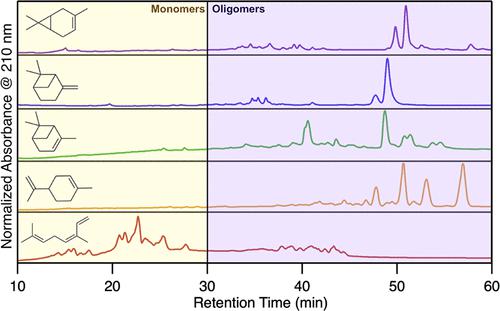
Biogenic sources dominate annual emissions of volatile organic compounds (VOCs) to the atmosphere. A large fraction of these are monoterpenes, which react with OH radicals, NO3 radicals, or O3 to form oxidized products, some of which partition to particles as secondary organic aerosol (SOA). Here, we compare the results of studies of the reaction of NO3 radicals, a nighttime oxidant, with five monoterpenes: Δ-3-carene, β-pinene, α-pinene, limonene, and ocimene. Whereas all of these monoterpenes have the molecular formula C10H16, they differ by having 1, 2, or 3 C═C double bonds and 0, 1, or 2 rings. Experiments were conducted in an environmental chamber under conditions in which RO2• + RO2• reactions were dominant, and gas- and particle-phase products were analyzed using mass spectrometry, gas and liquid chromatography, infrared spectroscopy, and derivatization-spectrophotometric methods. Gas-phase products were first-generation compounds with 2–4 functional groups, whereas SOA products were mostly acetal and hemiacetal dimers formed by particle-phase accretion reactions. The large contribution of dimers formed from hydroxycarbonyl nitrate and hydroxynitrate monomers indicates that they might be used as atmospheric tracers for NO3 radical-initiated reactions of monoterpenes. Conversely, gas-phase formation of ROOR dimers was negligible. Functional group analysis of SOA indicated ∼1 nitrate, ∼0.2–0.7 carbonyl groups, and ∼0–0.4 hydroxyl, carboxyl, ester, and peroxide groups per C10 product for all the monoterpenes. SOA mass yields were 56, 89, 48, 78, and 69% for Δ-3-carene, β-pinene, α-pinene, limonene, and ocimene, which combined with functional group analysis gives lower-limit estimates of organic nitrate yields of 34, 56, 35, 50, and 40%. Results were used to develop reaction mechanisms to explain the formation of gas- and particle-phase products and provide improved understanding of the role of molecular structure in VOC oxidation and particle-phase accretion reactions.
中文翻译:

NO3自由基引发的环状和非环状单萜氧化生成二次有机气溶胶的产物和机理
生物源主导着每年向大气排放的挥发性有机化合物 (VOC)。其中很大一部分是单萜,它们与 OH 自由基、NO 3自由基或 O 3反应形成氧化产物,其中一些以二次有机气溶胶 (SOA) 的形式分配到颗粒中。在这里,我们比较了 NO 3自由基(一种夜间氧化剂)与五种单萜反应的研究结果:Δ-3-蒎烯、β-蒎烯、α-蒎烯、柠檬烯和罗勒烯。尽管所有这些单萜都具有分子式 C 10 H 16,但它们的不同之处在于具有 1、2 或 3 个 C=C 双键和 0、1 或 2 个环。实验在 RO 2条件下的环境室中进行• + RO 2 • 反应占主导地位,使用质谱、气相和液相色谱、红外光谱和衍生分光光度法分析气相和颗粒相产物。气相产物是具有 2-4 个官能团的第一代化合物,而 SOA 产物主要是由颗粒相吸积反应形成的缩醛和半缩醛二聚体。由硝酸羟基羰基酯和羟基硝酸酯单体形成的二聚体的巨大贡献表明它们可以用作 NO 3的大气示踪剂单萜的自由基引发反应。相反,ROOR 二聚体的气相形成可以忽略不计。SOA 的官能团分析表明,对于所有单萜,每个 C 10产物有1 个硝酸盐、0.2-0.7 个羰基和 0-0.4 个羟基、羧基、酯和过氧化物基团。Δ-3-蒎烯、β-蒎烯、α-蒎烯、柠檬烯和罗勒烯的 SOA 质量产率为 56%、89%、48%、78% 和 69%,结合官能团分析得出有机硝酸盐产率的下限估计值34、56、35、50 和 40%。结果被用来开发反应机制来解释气相和颗粒相产物的形成,并更好地理解分子结构在 VOC 氧化和颗粒相吸积反应中的作用。
更新日期:2022-07-26
中文翻译:

NO3自由基引发的环状和非环状单萜氧化生成二次有机气溶胶的产物和机理
生物源主导着每年向大气排放的挥发性有机化合物 (VOC)。其中很大一部分是单萜,它们与 OH 自由基、NO 3自由基或 O 3反应形成氧化产物,其中一些以二次有机气溶胶 (SOA) 的形式分配到颗粒中。在这里,我们比较了 NO 3自由基(一种夜间氧化剂)与五种单萜反应的研究结果:Δ-3-蒎烯、β-蒎烯、α-蒎烯、柠檬烯和罗勒烯。尽管所有这些单萜都具有分子式 C 10 H 16,但它们的不同之处在于具有 1、2 或 3 个 C=C 双键和 0、1 或 2 个环。实验在 RO 2条件下的环境室中进行• + RO 2 • 反应占主导地位,使用质谱、气相和液相色谱、红外光谱和衍生分光光度法分析气相和颗粒相产物。气相产物是具有 2-4 个官能团的第一代化合物,而 SOA 产物主要是由颗粒相吸积反应形成的缩醛和半缩醛二聚体。由硝酸羟基羰基酯和羟基硝酸酯单体形成的二聚体的巨大贡献表明它们可以用作 NO 3的大气示踪剂单萜的自由基引发反应。相反,ROOR 二聚体的气相形成可以忽略不计。SOA 的官能团分析表明,对于所有单萜,每个 C 10产物有1 个硝酸盐、0.2-0.7 个羰基和 0-0.4 个羟基、羧基、酯和过氧化物基团。Δ-3-蒎烯、β-蒎烯、α-蒎烯、柠檬烯和罗勒烯的 SOA 质量产率为 56%、89%、48%、78% 和 69%,结合官能团分析得出有机硝酸盐产率的下限估计值34、56、35、50 和 40%。结果被用来开发反应机制来解释气相和颗粒相产物的形成,并更好地理解分子结构在 VOC 氧化和颗粒相吸积反应中的作用。































 京公网安备 11010802027423号
京公网安备 11010802027423号