当前位置:
X-MOL 学术
›
Chem. Soc. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electro-/photocatalytic alkene-derived radical cation chemistry: recent advances in synthetic applications
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2022-07-26 , DOI: 10.1039/d2cs00013j Mu-Jia Luo 1 , Qiang Xiao 1 , Jin-Heng Li 2, 3, 4
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2022-07-26 , DOI: 10.1039/d2cs00013j Mu-Jia Luo 1 , Qiang Xiao 1 , Jin-Heng Li 2, 3, 4
Affiliation
|
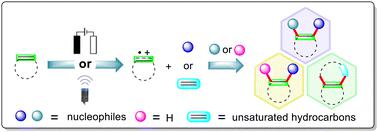
Alkene-derived radical cations are versatile reactive intermediates and have been widely applied in the construction of complex functionalized molecules and cyclic systems for chemical synthesis. Therefore, the synthetic application of these alkene-derived radical cations represents a powerful and green tool that can be used to achieve the functionalization of alkenes partially because the necessity of stoichiometric external chemical oxidants and/or hazardous reaction conditions is eliminated. This review summarizes the recent advances in the synthetic applications of the electro-/photochemical alkene-derived radical cations, emphasizing the key single-electron oxidation steps of the alkenes, the scope and limitations of the substrates, and the related reaction mechanisms. Using electrocatalysis and/or photocatalysis, single electron transfer (SET) oxidation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in the alkenes occurs, generating the alkene-derived radical cations, which sequentially enables the functionalization of translocated radical cations to occur in two ways: the first involves direct reaction with a nucleophile/radical or two molecules of nucleophiles to realize hydrofunctionalization, difunctionalization and cyclization; and the second involves the transformation of the alkene-derived radical cations into carbon-centered radicals using a base followed by radical coupling or oxidative nucleophilic coupling.
C bonds in the alkenes occurs, generating the alkene-derived radical cations, which sequentially enables the functionalization of translocated radical cations to occur in two ways: the first involves direct reaction with a nucleophile/radical or two molecules of nucleophiles to realize hydrofunctionalization, difunctionalization and cyclization; and the second involves the transformation of the alkene-derived radical cations into carbon-centered radicals using a base followed by radical coupling or oxidative nucleophilic coupling.
中文翻译:

电/光催化烯烃衍生自由基阳离子化学:合成应用的最新进展
烯烃衍生的自由基阳离子是通用的反应中间体,已广泛应用于构建复杂的功能化分子和化学合成的环状体系。因此,这些烯烃衍生自由基阳离子的合成应用代表了一种强大且绿色的工具,可用于实现烯烃的官能化,部分原因是消除了化学计量外部化学氧化剂和/或危险反应条件的必要性。本综述总结了电/光化学烯烃衍生自由基阳离子合成应用的最新进展,重点介绍了烯烃的关键单电子氧化步骤、底物的范围和局限性以及相关的反应机理。使用电催化和/或光催化,![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) 烯烃中的 C 键发生,生成烯烃衍生的自由基阳离子,从而使转移的自由基阳离子的官能化以两种方式发生:第一种涉及与亲核试剂/自由基或两个亲核试剂分子直接反应,以实现氢官能化、双官能化和环化;第二个涉及使用碱基将烯烃衍生的自由基阳离子转化为以碳为中心的自由基,然后进行自由基偶联或氧化亲核偶联。
烯烃中的 C 键发生,生成烯烃衍生的自由基阳离子,从而使转移的自由基阳离子的官能化以两种方式发生:第一种涉及与亲核试剂/自由基或两个亲核试剂分子直接反应,以实现氢官能化、双官能化和环化;第二个涉及使用碱基将烯烃衍生的自由基阳离子转化为以碳为中心的自由基,然后进行自由基偶联或氧化亲核偶联。
更新日期:2022-07-26
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in the alkenes occurs, generating the alkene-derived radical cations, which sequentially enables the functionalization of translocated radical cations to occur in two ways: the first involves direct reaction with a nucleophile/radical or two molecules of nucleophiles to realize hydrofunctionalization, difunctionalization and cyclization; and the second involves the transformation of the alkene-derived radical cations into carbon-centered radicals using a base followed by radical coupling or oxidative nucleophilic coupling.
C bonds in the alkenes occurs, generating the alkene-derived radical cations, which sequentially enables the functionalization of translocated radical cations to occur in two ways: the first involves direct reaction with a nucleophile/radical or two molecules of nucleophiles to realize hydrofunctionalization, difunctionalization and cyclization; and the second involves the transformation of the alkene-derived radical cations into carbon-centered radicals using a base followed by radical coupling or oxidative nucleophilic coupling.
中文翻译:

电/光催化烯烃衍生自由基阳离子化学:合成应用的最新进展
烯烃衍生的自由基阳离子是通用的反应中间体,已广泛应用于构建复杂的功能化分子和化学合成的环状体系。因此,这些烯烃衍生自由基阳离子的合成应用代表了一种强大且绿色的工具,可用于实现烯烃的官能化,部分原因是消除了化学计量外部化学氧化剂和/或危险反应条件的必要性。本综述总结了电/光化学烯烃衍生自由基阳离子合成应用的最新进展,重点介绍了烯烃的关键单电子氧化步骤、底物的范围和局限性以及相关的反应机理。使用电催化和/或光催化,
![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) 烯烃中的 C 键发生,生成烯烃衍生的自由基阳离子,从而使转移的自由基阳离子的官能化以两种方式发生:第一种涉及与亲核试剂/自由基或两个亲核试剂分子直接反应,以实现氢官能化、双官能化和环化;第二个涉及使用碱基将烯烃衍生的自由基阳离子转化为以碳为中心的自由基,然后进行自由基偶联或氧化亲核偶联。
烯烃中的 C 键发生,生成烯烃衍生的自由基阳离子,从而使转移的自由基阳离子的官能化以两种方式发生:第一种涉及与亲核试剂/自由基或两个亲核试剂分子直接反应,以实现氢官能化、双官能化和环化;第二个涉及使用碱基将烯烃衍生的自由基阳离子转化为以碳为中心的自由基,然后进行自由基偶联或氧化亲核偶联。










































 京公网安备 11010802027423号
京公网安备 11010802027423号