当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pd-Catalyzed asymmetric decarboxylation for the construction of spiro[4.5]deca-6,9-dien-8-ones featuring vicinal quaternary carbons
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-07-26 , DOI: 10.1039/d2qo00831a Haiyu Sun 1 , Yicheng He 1 , Wusheng Guo 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-07-26 , DOI: 10.1039/d2qo00831a Haiyu Sun 1 , Yicheng He 1 , Wusheng Guo 1
Affiliation
|
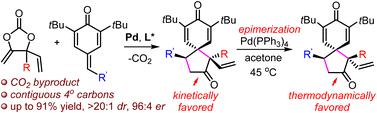
A Pd-catalyzed decarboxylative strategy for the asymmetric construction of spiro[4.5]deca-6,9-dien-8-ones is reported. This approach utilizes modular vinyl methylene cyclic carbonates and p-quinone methides as reaction partners. The reaction could be performed at room temperature and generates CO2 as the sole by-product. The corresponding products feature otherwise synthetically challenging vicinal quaternary carbons, which create great potential for complexity and diversity. The stereochemistry of the reactions is controlled with diastereo- and enantioselectivity being up to >20 : 1 dr and 96 : 4 er.
中文翻译:

Pd催化不对称脱羧构建具有连位季碳的螺[4.5]deca-6,9-dien-8-ones
报道了一种用于不对称构建螺[4.5]deca-6,9-dien-8-ones的Pd催化脱羧策略。该方法利用模块化乙烯基亚甲基环状碳酸酯和对醌甲基化物作为反应伙伴。该反应可以在室温下进行并产生CO 2作为唯一的副产物。相应的产品具有其他合成具有挑战性的连位季碳,这为复杂性和多样性创造了巨大的潜力。反应的立体化学受到控制,非对映选择性和对映选择性高达 >20:1 dr 和 96:4 er。
更新日期:2022-07-26
中文翻译:

Pd催化不对称脱羧构建具有连位季碳的螺[4.5]deca-6,9-dien-8-ones
报道了一种用于不对称构建螺[4.5]deca-6,9-dien-8-ones的Pd催化脱羧策略。该方法利用模块化乙烯基亚甲基环状碳酸酯和对醌甲基化物作为反应伙伴。该反应可以在室温下进行并产生CO 2作为唯一的副产物。相应的产品具有其他合成具有挑战性的连位季碳,这为复杂性和多样性创造了巨大的潜力。反应的立体化学受到控制,非对映选择性和对映选择性高达 >20:1 dr 和 96:4 er。































 京公网安备 11010802027423号
京公网安备 11010802027423号