Solar Energy ( IF 6.0 ) Pub Date : 2022-07-21 , DOI: 10.1016/j.solener.2022.07.023 Natalia Mazur , Henk Huinink , Hartmut Fischer , Pim Donkers , Olaf Adan
|
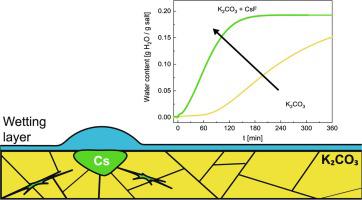
Potassium carbonate (K2CO3) is a promising thermochemical heat storage material (TCM). However, it suffers from hysteresis between (de)hydration temperatures and poor reaction kinetics close to equilibrium conditions. Both aspects are caused by a nucleation barrier and low ionic mobility close to equilibrium. This study investigates the impact of caesium fluoride (CsF) incorporated through recrystallisation on the phase transitions. The composition studies show that K2CO3 and CsF react during synthesis, forming KF, which points to the formation of Cs2CO3. The secondary phases are not incorporated into the crystal structure but reside between the main phase's grain cracks due to capillary forces. Because the secondary phases are highly hygroscopic, they promote surface mobility by forming a liquid-like layer even at low water vapour pressures. As the effect of their presence, hydration kinetics are enhanced significantly in all investigated conditions, with the most pronounced impact when hydration of K2CO3 is inherently inhibited. The benefits manifest themselves through a faster reaction rate and shorter induction period. The dehydration is enhanced by the presence of the additive mainly far away from equilibrium conditions. Close to the equilibrium, the dehydration of the composite proceeds in an unusual 2-step manner, where the second step is much slower than the dehydration of pure K2CO3. The enhancement of dehydration kinetics is ascribed to the formation of defects during recrystallisation. The lowering of dehydration rates close to equilibrium is attributed to diffusion issues due to excess of a deliquescent phase present in the system.
中文翻译:

从热化学蓄热角度通过添加 CsF 加速 K2CO3 的反应动力学
碳酸钾(K 2 CO 3)是一种很有前途的热化学蓄热材料(TCM)。然而,它在(脱水)水合温度和接近平衡条件的不良反应动力学之间存在滞后现象。这两个方面都是由成核势垒和接近平衡的低离子迁移率引起的。本研究调查了通过重结晶掺入的氟化铯 (CsF) 对相变的影响。成分研究 表明,K 2 CO 3 和 CsF 在合成过程中发生反应,形成 KF,这表明形成了 Cs 2 CO 3. 次生相没有结合到晶体结构中,但由于毛细作用力而存在于主相的晶粒裂缝之间。由于第二相具有高度吸湿性,因此即使在低水蒸气压力下,它们也会通过形成类似液体的层来促进表面流动性。由于它们的存在,水合动力学在所有研究条件下都显着增强,当 K 2 CO 3 的水合受到固有抑制时影响最为显着。这些好处 通过更快的反应速度和更短的诱导期体现出来。 主要远离平衡条件的添加剂的存在增强了脱水。接近平衡时,复合材料的脱水以不寻常的两步方式进行,其中第二步比纯 K 2 CO 3的脱水慢得多。脱水动力学的增强归因于再结晶过程中缺陷的形成。接近平衡的脱水率降低归因于由于系统中存在过量潮解相而引起的扩散问题。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号