CardioVascular and Interventional Radiology ( IF 2.8 ) Pub Date : 2022-07-21 , DOI: 10.1007/s00270-022-03224-w Jung Guen Cha 1 , Jongmin Lee 1 , Sang Yub Lee 1 , Ho Yun Chung 2 , Seok Jong Lee 3 , Seung Huh 4 , Ji Yoon Kim 5 , Jihoon Hong 1
|
Purpose
To evaluate the safety and efficacy of bleomycin infusion sclerotherapy using a syringe pump in microcystic and mixed (microcystic components with the presence of a cyst over 1 cm) lymphatic malformations (LMs).
Materials and Methods
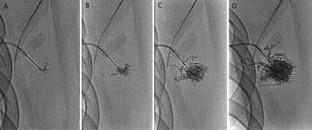
Patients who received bleomycin sclerotherapy with a syringe pump for microcystic or mixed LMs were reviewed. Cystic components of LMs were accessed under sonographic guidance, followed by injection of an opacified bleomycin solution using a syringe pump (infusion rate, 10–20 mL/h) under fluoroscopic guidance. Imaging outcomes were graded as complete (> 90% size reduction), partial (25–90%), or no response (< 25%). Clinical outcomes and procedure-related complications were also reviewed.
Results
Forty-nine patients with 81 sclerotherapies were analyzed. The mean age was 17 years (range 0.1–65 y). Thirty-one (63%) patients had microcystic LMs, and 18 (37%) had mixed. A mean of 1.7 sessions (range 1–4) of sclerotherapy was performed using a mean cumulative dose of bleomycin of 10.8 U (range 1.5–39 U). The mean infusion time was 39 min (range 14–130 min). Regarding imaging outcomes, there was a complete response in 29% (n = 14), a partial response in 57% (n = 28), and no response in 14% (n = 7). Regarding clinical outcomes, there was a complete response in 39% (n = 19), a partial response in 51% (n = 25), and no response in 10% (n = 5). According to the CIRSE classification, no major complications were identified.
Conclusions
Bleomycin slow infusion sclerotherapy provides gradual filling of sclerosant to target microcystic components. This technique is safe and feasible for the management of microcystic or mixed LMs.
Level of Evidence
Level 4, Case series.
中文翻译:

使用注射泵进行博莱霉素缓慢输注硬化治疗微囊性和混合性淋巴管畸形的安全性和有效性
目的
评估使用注射泵对微囊性和混合性(微囊性成分,囊肿超过 1 厘米)淋巴畸形 (LMs) 进行博莱霉素输注硬化疗法的安全性和有效性。
材料和方法
回顾了使用注射泵治疗微囊或混合 LM 的博来霉素硬化疗法的患者。在超声引导下获取 LMs 的囊性成分,然后在透视引导下使用注射泵(输注速度,10-20 mL/h)注射不透明的博来霉素溶液。成像结果分为完全(> 90% 尺寸减小)、部分(25-90%)或无反应(<25%)。还审查了临床结果和与手术相关的并发症。
结果
对 81 种硬化疗法的 49 名患者进行了分析。平均年龄为 17 岁(范围 0.1-65 岁)。31 名 (63%) 患者患有微囊性 LM,18 名 (37%) 患者为混合型。使用平均累积剂量为 10.8 U(范围 1.5-39 U)的博来霉素进行平均 1.7 次(范围 1-4)的硬化治疗。平均输注时间为 39 分钟(范围 14-130 分钟)。关于成像结果,29% ( n = 14) 有完全反应,57% ( n = 28) 有部分反应,14% ( n = 7) 没有反应。关于临床结果,39% ( n = 19) 完全缓解,51% ( n = 25) 部分缓解,10% ( n = = 5)。根据 CIRSE 分类,未发现重大并发症。
结论
博来霉素缓慢输注硬化疗法提供硬化剂逐渐填充以靶向微囊成分。该技术对于微囊性或混合性 LM 的管理是安全可行的。
证据水平
4 级,案例系列。

































 京公网安备 11010802027423号
京公网安备 11010802027423号