当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Demarcating Noncovalent and Covalent Bond Territories: Imine-CO2 Complexes and Cooperative CO2 Capture
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2022-07-21 , DOI: 10.1021/acs.jpca.2c03221 Sebastian Anila 1, 2 , Cherumuttathu H Suresh 1, 2 , Henry F Schaefer 3
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2022-07-21 , DOI: 10.1021/acs.jpca.2c03221 Sebastian Anila 1, 2 , Cherumuttathu H Suresh 1, 2 , Henry F Schaefer 3
Affiliation
|
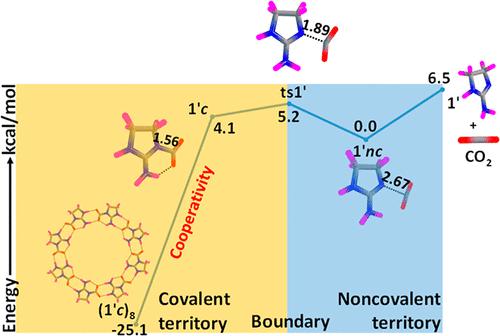
Chemical bond territory is rich with covalently bonded molecules wherein a strong bond is formed by equal or unequal sharing of a quantum of electrons. The noncovalent version of the bonding scenarios expands the chemical bonding territory to a weak domain wherein the interplay of electrostatic and π-effects, dipole–dipole, dipole–induced dipole, and induced dipole–induced dipole interactions, and hydrophobic effects occur. Here we study both the covalent and noncovalent interactive behavior of cyclic and acyclic imine-based functional molecules (XN) with CO2. All parent XN systems preferred the formation of noncovalent (nc) complex XN···CO2, while more saturated such systems (XN′) produced both nc and covalent (c) complexes XN′+–(CO2)−. In all such cases, crossover from an nc to c complex is clearly demarcated with the identification of a transition state (ts). The complexes XN′···CO2 and XN′+–(CO2)− are bond stretch isomers, and they define the weak and strong bonding territories, respectively, while the ts appears as the demarcation point of the two territories. Cluster formation of XN with CO2 reinforces the interaction between them, and all become covalent clusters of general formula (XN+–(CO2)−)n. The positive cooperativity associated with the NH···OC hydrogen bond formation between any two XN′+–(CO2)− units strengthened the N–C coordinate covalent bond and led to massive stabilization of the cluster. For instance, the stabilizing interaction between the XN unit with CO2 is increased from 2–7 kcal/mol range in a monomer complex to 14–31 kcal/mol range for the octamer cluster (XN′+–(CO2)−)8. The cooperativity effect compensates for the large reduction in the entropy of cluster formation. Several imine systems showed the exergonic formation of the cluster and are predicted as potential candidates for CO2 capture and conversion.
中文翻译:

划定非共价键和共价键区域:亚胺-CO2 配合物和 CO2 协同捕获
化学键区域富含共价键分子,其中强键是由相等或不相等的电子量子共享形成的。键合场景的非共价形式将化学键合区域扩展到弱域,其中静电和 π 效应、偶极-偶极、偶极诱导偶极和诱导偶极诱导偶极相互作用以及疏水效应的相互作用发生。在这里,我们研究了环状和非环状亚胺基功能分子 (XN) 与 CO 2的共价和非共价相互作用行为。所有母 XN 系统都倾向于形成非共价 ( nc ) 复合物 XN···CO 2,而更饱和的此类系统 (XN') 则同时产生nc和共价 ( c) 配合物 XN' + –(CO 2 ) -。在所有这些情况下,从nc到c复合体的交叉通过识别过渡状态 ( ts ) 明确划分。配合物XN'···CO 2和XN' + -(CO 2 ) -是键伸缩异构体,它们分别定义了弱键区和强键区,而ts则作为这两个键区的分界点。XN与CO 2的团簇形成加强了它们之间的相互作用,都成为通式(XN + –(CO 2) - ) n。与任意两个XN' + -(CO 2 ) -单元之间的NH···OC 氢键形成相关的正协同作用加强了N-C 配位共价键并导致簇的大量稳定。例如,XN 单元与 CO 2之间的稳定相互作用从单体配合物中的 2-7 kcal/mol 范围增加到八聚体簇 (XN' + –(CO 2 ) - )的 14-31 kcal/mol 范围8. 协同效应补偿了簇形成熵的大幅降低。几个亚胺系统显示了该簇的放能形成,并被预测为CO 2捕获和转化的潜在候选者。
更新日期:2022-07-21
中文翻译:

划定非共价键和共价键区域:亚胺-CO2 配合物和 CO2 协同捕获
化学键区域富含共价键分子,其中强键是由相等或不相等的电子量子共享形成的。键合场景的非共价形式将化学键合区域扩展到弱域,其中静电和 π 效应、偶极-偶极、偶极诱导偶极和诱导偶极诱导偶极相互作用以及疏水效应的相互作用发生。在这里,我们研究了环状和非环状亚胺基功能分子 (XN) 与 CO 2的共价和非共价相互作用行为。所有母 XN 系统都倾向于形成非共价 ( nc ) 复合物 XN···CO 2,而更饱和的此类系统 (XN') 则同时产生nc和共价 ( c) 配合物 XN' + –(CO 2 ) -。在所有这些情况下,从nc到c复合体的交叉通过识别过渡状态 ( ts ) 明确划分。配合物XN'···CO 2和XN' + -(CO 2 ) -是键伸缩异构体,它们分别定义了弱键区和强键区,而ts则作为这两个键区的分界点。XN与CO 2的团簇形成加强了它们之间的相互作用,都成为通式(XN + –(CO 2) - ) n。与任意两个XN' + -(CO 2 ) -单元之间的NH···OC 氢键形成相关的正协同作用加强了N-C 配位共价键并导致簇的大量稳定。例如,XN 单元与 CO 2之间的稳定相互作用从单体配合物中的 2-7 kcal/mol 范围增加到八聚体簇 (XN' + –(CO 2 ) - )的 14-31 kcal/mol 范围8. 协同效应补偿了簇形成熵的大幅降低。几个亚胺系统显示了该簇的放能形成,并被预测为CO 2捕获和转化的潜在候选者。














































 京公网安备 11010802027423号
京公网安备 11010802027423号