Journal of Solid State Chemistry ( IF 3.2 ) Pub Date : 2022-07-21 , DOI: 10.1016/j.jssc.2022.123438
S.А. Osseni , P.O. Andreev , A.A. Polkovnikov , B.A. Zakharov , A.S. Aleksandrovsky , M.U. Abulkhaev , S.S. Volkova , D.N. Kamaev , I.M. Kovenskiy , N.V. Nesterova , M.V. Kudomanov , O.V. Andreev
|
我们已经确定了 Nd 2 S 3 - Nd 2 O 3体系中硫化物和氧硫化物相的热特性和光学性质。
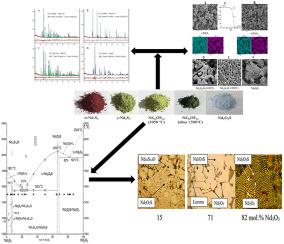
通过 DSC 方法检测到 Nd 2 S 3化合物在温度 1801 ± 4.9 °C 处具有一致的熔融峰,Δ H = 65.2 ± 6.7 kJ/mol 。α-Nd 2 S 3 → γ-Nd 2 S 3多晶型跃迁的特征是t = 1183 ± 1.8°С,ΔH = 7.5 ± 0.3 kJ/mol。800℃退火时冷却得到的γ-Nd 2 S 3相最多保留30 h,然后在20 h内发生γ-Nd 2 S 3 →α-Nd 2 S 3转变。相的显微硬度为:α-Nd 2 S3 H = 451 ± 4 HV;γ-Nd 2 S 3 H = 531 ± 4 HV。
TG法发现Nd 10 S 14 O相在1400℃以上发生热解离。质量损失在 1580 ℃时为 0.5 质量%,在 1620 ℃时为 1.0 质量%,但样品在冷却后仍为单相。然而,在高于 1620 ± 20 °C 的温度下处理的 Nd 10 S 14 O 样品中出现了两个杂质相 γ-Nd 2 S 3-X和 Nd 2 O 2 S。
对于在 1050、1400、1580 °C 温度下在氩气氛中退火的 Nd 10 S 14 O 相样品,记录到晶胞参数和光学带隙有规律的降低:1050 °C a = 15.06291(28) , c = 19.97864(35), E g = 2, 63 eV, 1400 °C a = 15.04779(36), c = 19.97160(44), E g = 2.64 eV; 1580 °C a = 15.03532(48),c = 19.94984(60),E g = 2.51 eV。Nd 10 S 14 O的显微硬度为H = 549 ± 10 HV。Nd 2O 2 S 相具有H = 593 ± 4 HV,E g = 4.28 eV。
构建了Nd2S3-Nd2O3体系从1000℃到熔体的相图。Nd2O2S 相在 2050 ± 30 °C 时完全熔化。共晶坐标 23 mol。% Nd2O3 (0.3484 Nd10S14O + 0.6516 Nd2O2S), t = 1553 ± 1.8°С; ΔH = 187 ± 19 焦耳/克;82 摩尔。% Nd2O3; (0.54 Nd2O2S + 0.46 Nd2O3), t = 1970 ± 30°С。Nd2S3-Nd2O3 体系的液相线是根据 DSC 数据建立的,并使用 Redlich-Kister 方程计算。使用 Schroeder 方程计算 Nd 2 O 2 S ΔH = 67 kJ/mol的熔化焓。

"点击查看英文标题和摘要"

































 京公网安备 11010802027423号
京公网安备 11010802027423号