Dyes and Pigments ( IF 4.1 ) Pub Date : 2022-07-20 , DOI: 10.1016/j.dyepig.2022.110592 Tatyana N. Moshkina , Emiliya V. Nosova , Julia V. Permyakova , Galina N. Lipunova , Marina S. Valova , Pavel A. Slepukhin , Leila K. Sadieva , Valery N. Charushin
|
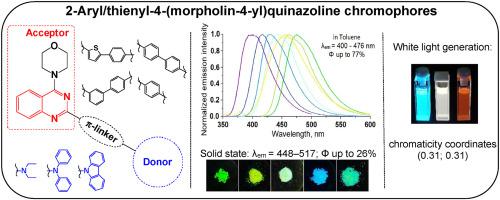
A few-stages synthetic approach to novel 2-aryl-4-(morpholin-4-yl)quinazolines has been proposed. The photophysical properties of target compounds have been investigated in two solvents and compared with those for 2-thienyl-4-(morpholin-4-yl)quinazoline counterparts. The influence of nature, arrangement, and the length of π-linker moiety on the photophysical characteristics of dyes have been analyzed. In general, the replacement of thienylene ring with phenylene one results in blue-shifted absorption and emission maxima, whereas the compound with shortened π-system exhibits slight changes in both absorption and emission in toluene solution compared to the corresponding bi-phenylene counterpart. The compounds emit in solutions with quantum yield up to 75% in toluene and up to 46% in acetonitrile. Fluorescence colour and intensity change upon gradual addition of acid to the solution of the chromophore in toluene. It was found that (5-(4-diphenylaminophenyl)thiophen-2-yl)-4-(morpholin-4-yl)quinazoline is capable of generating white luminescence. Additionally, emission behaviour of quinazolines in the MeCN/water mixtures with different water content has been studied. The changes in position and intensity of the emission band were noted for all the compounds upon addition of water to the solution of quinazoline in MeCN; some chromophores show enhanced emission at 70–80 vol% of water fraction. Finally, 4-morpholinyl chromophores exhibit emission in the solid state. The biphenylene quinazolines emit deep-blue light in powder, while 2-(4-diphenylamino)phenylquinazoline and 2-(5-aminoaryl)thiophen-2-yl counterparts possess green or blue-green fluorescence.
中文翻译:

2-aryl-4-(morpholin-4-yl)quinazoline 发色团的合成和光物理性质:π-接头部分的影响
已经提出了新型 2-aryl-4-(morpholin-4-yl) 喹唑啉的几步合成方法。已经在两种溶剂中研究了目标化合物的光物理性质,并与 2-噻吩基-4-(morpholin-4-yl) 喹唑啉对应物的光物理性质进行了比较。分析了π-接头部分的性质、排列和长度对染料光物理特性的影响。一般来说,用亚苯基一取代亚噻吩环会导致吸收和发射最大值蓝移,而具有缩短 π 系统的化合物在甲苯溶液中的吸收和发射与相应的联苯对应物相比表现出轻微的变化。这些化合物在溶液中以量子产率发光在甲苯中高达 75%,在乙腈中高达 46%。逐渐向溶液中加入酸后,荧光颜色和强度会发生变化甲苯中的发色团。发现(5-(4-二苯基氨基苯基)噻吩-2-基)-4-(吗啉-4-基)喹唑啉能够产生白光。此外,还研究了喹唑啉在不同含水量的 MeCN/水混合物中的排放行为。将水加入到喹唑啉的 MeCN 溶液中后,所有化合物的发射带位置和强度都发生了变化;一些发色团在 70–80 vol% 的水份中显示出增强的发射。最后,4-吗啉基发色团在固态显示发射。联苯喹唑啉在粉末中发出深蓝色光,而 2-(4-二苯氨基)苯基喹唑啉和 2-(5-氨基芳基)噻吩-2-基对应物具有绿色或蓝绿色荧光。

































 京公网安备 11010802027423号
京公网安备 11010802027423号