当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iodine–Iodine Cooperation Enables Metal-Free C–N Bond-Forming Electrocatalysis via Isolable Iodanyl Radicals
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-07-20 , DOI: 10.1021/jacs.2c05562 Brandon L Frey 1 , Matthew T Figgins 1 , Gerard P Van Trieste 1 , Raanan Carmieli 2 , David C Powers 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-07-20 , DOI: 10.1021/jacs.2c05562 Brandon L Frey 1 , Matthew T Figgins 1 , Gerard P Van Trieste 1 , Raanan Carmieli 2 , David C Powers 1
Affiliation
|
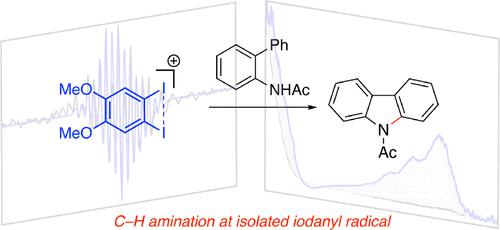
Small molecule redox mediators convey interfacial electron transfer events into bulk solution and can enable diverse substrate activation mechanisms in synthetic electrocatalysis. Here, we report that 1,2-diiodo-4,5-dimethoxybenzene is an efficient electrocatalyst for C–H/E–H coupling that operates at as low as 0.5 mol % catalyst loading. Spectroscopic, crystallographic, and computational results indicate a critical role for a three-electron I–I bonding interaction in stabilizing an iodanyl radical intermediate (i.e., formally I(II) species). As a result, the optimized catalyst operates at more than 100 mV lower potential than the related monoiodide catalyst 4-iodoanisole, which results in improved product yield, higher Faradaic efficiency, and expanded substrate scope. The isolated iodanyl radical is chemically competent in C–N bond formation. These results represent the first examples of substrate functionalization at a well-defined I(II) derivative and bona fide iodanyl radical catalysis and demonstrate one-electron pathways as a mechanistic alternative to canonical two-electron hypervalent iodine mechanisms. The observation establishes I–I redox cooperation as a new design concept for the development of metal-free redox mediators.
中文翻译:

碘-碘合作通过可分离的碘自由基实现无金属 C-N 键形成电催化
小分子氧化还原介体将界面电子转移事件传递到本体溶液中,并可以在合成电催化中实现多种底物活化机制。在此,我们报道 1,2-二碘-4,5-二甲氧基苯是一种高效的 C-H/E-H 偶联电催化剂,其催化剂负载量低至 0.5 mol%。光谱、晶体学和计算结果表明三电子 I-I 键合相互作用在稳定碘自由基中间体(即,正式为 I(II) 物种)。因此,优化后的催化剂的工作电位比相关的一碘化物催化剂 4-碘苯甲醚低 100 mV 以上,从而提高了产物收率、提高了法拉第效率并扩大了底物范围。孤立的碘基自由基在化学上能够形成 C-N 键。这些结果代表了在明确定义的 I(II) 衍生物和真正的碘基自由基催化下底物功能化的第一个例子,并证明单电子途径是典型双电子高价碘机制的机械替代方案。该观察结果将 I-I 氧化还原合作确立为开发无金属氧化还原介体的新设计概念。
更新日期:2022-07-20
中文翻译:

碘-碘合作通过可分离的碘自由基实现无金属 C-N 键形成电催化
小分子氧化还原介体将界面电子转移事件传递到本体溶液中,并可以在合成电催化中实现多种底物活化机制。在此,我们报道 1,2-二碘-4,5-二甲氧基苯是一种高效的 C-H/E-H 偶联电催化剂,其催化剂负载量低至 0.5 mol%。光谱、晶体学和计算结果表明三电子 I-I 键合相互作用在稳定碘自由基中间体(即,正式为 I(II) 物种)。因此,优化后的催化剂的工作电位比相关的一碘化物催化剂 4-碘苯甲醚低 100 mV 以上,从而提高了产物收率、提高了法拉第效率并扩大了底物范围。孤立的碘基自由基在化学上能够形成 C-N 键。这些结果代表了在明确定义的 I(II) 衍生物和真正的碘基自由基催化下底物功能化的第一个例子,并证明单电子途径是典型双电子高价碘机制的机械替代方案。该观察结果将 I-I 氧化还原合作确立为开发无金属氧化还原介体的新设计概念。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号