当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Construction, Pesticidal Activities, Control Effects, and Detoxification Enzyme Activities of Osthole Ester/Amide Derivatives
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2022-07-20 , DOI: 10.1021/acs.jafc.2c02534 Meng Hao 1 , Min Lv 1 , Lin Zhou 1 , Haijie Li 1 , Jianwei Xu 1 , Hui Xu 1, 2
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2022-07-20 , DOI: 10.1021/acs.jafc.2c02534 Meng Hao 1 , Min Lv 1 , Lin Zhou 1 , Haijie Li 1 , Jianwei Xu 1 , Hui Xu 1, 2
Affiliation
|
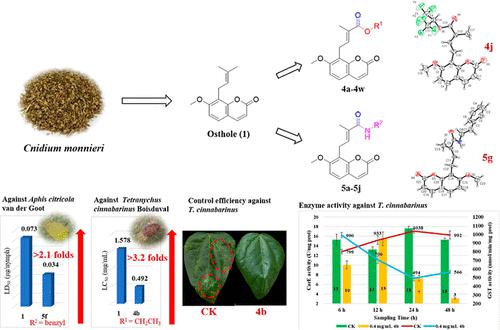
Pesticide research and development has entered an era of safety, efficiency, and environmental friendliness. Discovery of effective active products directly or indirectly from plant secondary metabolites as pesticide candidates has been one of the current research focuses. Herein, two series of new ester and amide derivatives were prepared by structural modifications of a natural coumarin-type product osthole at its C-4′ position. Their structures were characterized by IR, mp, 1H NMR, and HRMS. Confirmation of steric configuration of seven compounds was based on single-crystal analysis. Against Tetranychus cinnabarinus Boisduval (Acari: Tetranychidae), (2′E)-3′-ethoxycarbonylosthole (4b) and (2′E)-3′-(n)hexyloxycarbonylosthole (4e) exhibited 3.2 and 3.1 times acaricidal activity of osthole, and particularly, they also showed 2.4 and 2.2 times control efficiency on the 5th day of osthole. Against Aphis citricola Van der Goot (Homoptera: Aphididae), (2′E)-3′-(p-CF3)benzyloxycarbonylosthole (4w), (2′E)-3′-benzylaminocarbonylosthole (5f), and (2′E)-3′-phenylethylaminocarbonylosthole (5g) showed 1.9–2.1-fold aphicidal activity of osthole. Furthermore, the changes in two detoxification enzyme [carboxylesterase (CarE) and glutathione S-transferase (GST)] activities over time in treated T. cinnabarinus were investigated. These results can pave the foundation for future design and preparation of osthole derivatives as botanical agrochemicals.
中文翻译:

蛇床子素酯/酰胺衍生物的构建、杀虫活性、防治效果和解毒酶活性
农药研发已进入安全、高效、环保的时代。直接或间接从植物次生代谢产物中发现有效的活性产物作为农药候选物一直是当前的研究重点之一。在此,通过对天然香豆素型产物蛇床子素的 C-4' 位进行结构修饰,制备了两个系列的新型酯和酰胺衍生物。它们的结构通过 IR、mp、1 H NMR 和 HRMS 进行了表征。七种化合物的立体构型的确认是基于单晶分析。对抗朱砂叶螨(Acari: Tetranychidae), (2' E )-3'-ethoxycarbonylosthole ( 4b ) 和 (2' E )-3'-(n )己氧基羰基蛇床子素( 4e )的杀螨活性是蛇床子素的3.2倍和3.1倍,特别是在蛇床子素的第5天,它们的防治效率也分别为2.4和2.2倍。针对Aphis citricola Van der Goot (Homoptera: Aphididae)、(2' E )-3'-( p -CF 3 )benzyloxycarbonylosthole ( 4w )、(2' E )-3'-benzylaminocarbonylosthole ( 5f ) 和 (2' E )-3'-苯乙基氨基羰基蛇床子素 ( 5g ) 显示出蛇床子素的 1.9-2.1 倍的杀蚜活性。此外,两种解毒酶 [羧酸酯酶 (CarE) 和谷胱甘肽S研究了处理过的T. cinnabarinus中随时间推移的转移酶 (GST)] 活性。这些结果可以为未来设计和制备蛇床子素衍生物作为植物农用化学品奠定基础。
更新日期:2022-07-20
中文翻译:

蛇床子素酯/酰胺衍生物的构建、杀虫活性、防治效果和解毒酶活性
农药研发已进入安全、高效、环保的时代。直接或间接从植物次生代谢产物中发现有效的活性产物作为农药候选物一直是当前的研究重点之一。在此,通过对天然香豆素型产物蛇床子素的 C-4' 位进行结构修饰,制备了两个系列的新型酯和酰胺衍生物。它们的结构通过 IR、mp、1 H NMR 和 HRMS 进行了表征。七种化合物的立体构型的确认是基于单晶分析。对抗朱砂叶螨(Acari: Tetranychidae), (2' E )-3'-ethoxycarbonylosthole ( 4b ) 和 (2' E )-3'-(n )己氧基羰基蛇床子素( 4e )的杀螨活性是蛇床子素的3.2倍和3.1倍,特别是在蛇床子素的第5天,它们的防治效率也分别为2.4和2.2倍。针对Aphis citricola Van der Goot (Homoptera: Aphididae)、(2' E )-3'-( p -CF 3 )benzyloxycarbonylosthole ( 4w )、(2' E )-3'-benzylaminocarbonylosthole ( 5f ) 和 (2' E )-3'-苯乙基氨基羰基蛇床子素 ( 5g ) 显示出蛇床子素的 1.9-2.1 倍的杀蚜活性。此外,两种解毒酶 [羧酸酯酶 (CarE) 和谷胱甘肽S研究了处理过的T. cinnabarinus中随时间推移的转移酶 (GST)] 活性。这些结果可以为未来设计和制备蛇床子素衍生物作为植物农用化学品奠定基础。































 京公网安备 11010802027423号
京公网安备 11010802027423号