当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Heteroepitaxial Growth of B5-Site-Rich Ru Nanoparticles Guided by Hexagonal Boron Nitride for Low-Temperature Ammonia Dehydrogenation
Advanced Materials ( IF 27.4 ) Pub Date : 2022-07-19 , DOI: 10.1002/adma.202203364 Sungsu Kang 1, 2 , Junyoung Cha 3 , Young Suk Jo 3 , Yu-Jin Lee 3 , Hyuntae Sohn 3 , Younhwa Kim 1, 2 , Chyan Kyung Song 1, 2 , Yongmin Kim 3 , Dong-Hee Lim 4 , Jungwon Park 1, 2, 5, 6 , Chang Won Yoon 7, 8, 9
Advanced Materials ( IF 27.4 ) Pub Date : 2022-07-19 , DOI: 10.1002/adma.202203364 Sungsu Kang 1, 2 , Junyoung Cha 3 , Young Suk Jo 3 , Yu-Jin Lee 3 , Hyuntae Sohn 3 , Younhwa Kim 1, 2 , Chyan Kyung Song 1, 2 , Yongmin Kim 3 , Dong-Hee Lim 4 , Jungwon Park 1, 2, 5, 6 , Chang Won Yoon 7, 8, 9
Affiliation
|
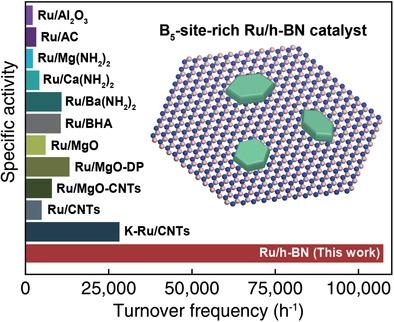
Ruthenium is one of the most active catalysts for ammonia dehydrogenation and is essential for the use of ammonia as a hydrogen storage material. The B5-type site on the surface of ruthenium is expected to exhibit the highest catalytic activity for ammonia dehydrogenation, but the number of these sites is typically low. Here, a B5-site-rich ruthenium catalyst is synthesized by exploiting the crystal symmetry of a hexagonal boron nitride support. In the prepared ruthenium catalyst, ruthenium nanoparticles are formed epitaxially on hexagonal boron nitride sheets with hexagonal planar morphologies, in which the B5 sites predominate along the nanoparticle edges. By activating the catalyst under the reaction condition, the population of B5 sites further increases as the facets of the ruthenium nanoparticles develop. The electron density of the Ru nanoparticles also increases during catalyst activation. The synthesized catalyst shows superior catalytic activity for ammonia dehydrogenation compared to previously reported catalysts. This work demonstrates that morphology control of a catalyst via support-driven heteroepitaxy can be exploited for synthesizing highly active heterogeneous catalysts with tailored atomic structures.
中文翻译:

六方氮化硼引导富 B5 位 Ru 纳米粒子的异质外延生长用于低温氨脱氢
钌是氨脱氢最活跃的催化剂之一,对于将氨用作储氢材料至关重要。预计钌表面上的 B 5型位点对氨脱氢表现出最高的催化活性,但这些位点的数量通常很少。在这里,通过利用六方氮化硼载体的晶体对称性合成了富含B 5位点的钌催化剂。在制备的钌催化剂中,钌纳米颗粒在具有六方平面形貌的六方氮化硼片上外延形成,其中B 5位点沿纳米颗粒边缘占优势。通过在反应条件下激活催化剂,B 5的数量随着钌纳米颗粒的面的发展,位点进一步增加。Ru 纳米粒子的电子密度也在催化剂活化过程中增加。与先前报道的催化剂相比,合成的催化剂对氨脱氢显示出优异的催化活性。这项工作表明,通过载体驱动的异质外延对催化剂的形态控制可用于合成具有定制原子结构的高活性多相催化剂。
更新日期:2022-07-19
中文翻译:

六方氮化硼引导富 B5 位 Ru 纳米粒子的异质外延生长用于低温氨脱氢
钌是氨脱氢最活跃的催化剂之一,对于将氨用作储氢材料至关重要。预计钌表面上的 B 5型位点对氨脱氢表现出最高的催化活性,但这些位点的数量通常很少。在这里,通过利用六方氮化硼载体的晶体对称性合成了富含B 5位点的钌催化剂。在制备的钌催化剂中,钌纳米颗粒在具有六方平面形貌的六方氮化硼片上外延形成,其中B 5位点沿纳米颗粒边缘占优势。通过在反应条件下激活催化剂,B 5的数量随着钌纳米颗粒的面的发展,位点进一步增加。Ru 纳米粒子的电子密度也在催化剂活化过程中增加。与先前报道的催化剂相比,合成的催化剂对氨脱氢显示出优异的催化活性。这项工作表明,通过载体驱动的异质外延对催化剂的形态控制可用于合成具有定制原子结构的高活性多相催化剂。

































 京公网安备 11010802027423号
京公网安备 11010802027423号