当前位置:
X-MOL 学术
›
Arch. Pharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of new N-(3-oxo-1-thia-4-azaspiro[4.5]decan-4-yl)pyridine-3-carboxamide derivatives and evaluation of their anti-influenza virus and antitubercular activities
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2022-07-18 , DOI: 10.1002/ardp.202200224 Gökçe Cihan-Üstündağ 1 , Çiğdem Acar 1 , Lieve Naesens 2 , Gonca Erköse-Genç 3 , Dilek Şatana 3
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2022-07-18 , DOI: 10.1002/ardp.202200224 Gökçe Cihan-Üstündağ 1 , Çiğdem Acar 1 , Lieve Naesens 2 , Gonca Erköse-Genç 3 , Dilek Şatana 3
Affiliation
|
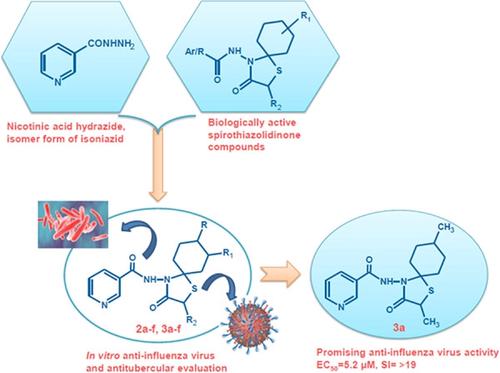
We here report the synthesis, structural characterization, and evaluation of the antiviral and antitubercular activities of a novel series of hybrid spirothiazolidinone derivatives (2a–f and 3a–f) containing the nicotinohydrazide moiety, which is an isomer form of the approved antitubercular drug isoniazid. When evaluated for activity against influenza A/H1N1, A/H3N2, and B viruses, three of the new compounds proved to possess specific antiviral activity against the influenza A/H3N2 virus. The most active analog 3a, bearing a 2,8-dimethyl group at the spiro ring, displayed an antiviral EC50 value of 5.2 µM. Compound 3a produced no cytotoxicity at 100 µM, the highest concentration tested, giving a selectivity index of at least 19. Structure–activity relationship analysis indicated that the absence of the methyl substituent at the 2-position and the presence of a bulky substituent at the 8-position of the spirothiazolidinone system caused a significant decrease in antiviral activity. The in vitro antitubercular activity of compounds 2a–f and 3a–f was determined for six different drug-sensitive/drug-resistant laboratory strains and clinical isolates of Mycobacterium tuberculosis. Compounds 2c, 2d, 3b, 3c, and 3d showed weak antitubercular activity against different strains, with MIC values of 125–250 μM.
中文翻译:

新型 N-(3-oxo-1-thia-4-azaspiro[4.5]decan-4-yl)pyridine-3-carboxamide 衍生物的合成及其抗流感病毒和抗结核活性的评价
我们在此报告了一系列新型杂合螺噻唑烷酮衍生物(2a-f和3a-f)的合成、结构表征和抗病毒和抗结核活性评估,该衍生物含有烟酰肼部分,这是已批准的抗结核药物异烟肼的异构体形式. 在评估对甲型/H1N1、A/H3N2 和 B 型流感病毒的活性时,三种新化合物证明对甲型/H3N2 流感病毒具有特异性抗病毒活性。最活跃的类似物3a,在螺环上带有 2,8-二甲基基团,显示出 5.2 µM 的抗病毒 EC 50值。化合物3a在 100 µM(测试的最高浓度)下没有产生细胞毒性,选择性指数至少为 19。构效关系分析表明,在 2 位不存在甲基取代基,而在 8 位存在庞大的取代基。螺噻唑烷酮系统的位置导致抗病毒活性显着降低。化合物2a-f和3a-f的体外抗结核活性对六种不同的药物敏感/耐药实验室菌株和结核分枝杆菌临床分离株进行了测定。化合物2c、2d、3b、3c和3d对不同菌株显示出较弱的抗结核活性,MIC 值为 125-250 μM。
更新日期:2022-07-18
中文翻译:

新型 N-(3-oxo-1-thia-4-azaspiro[4.5]decan-4-yl)pyridine-3-carboxamide 衍生物的合成及其抗流感病毒和抗结核活性的评价
我们在此报告了一系列新型杂合螺噻唑烷酮衍生物(2a-f和3a-f)的合成、结构表征和抗病毒和抗结核活性评估,该衍生物含有烟酰肼部分,这是已批准的抗结核药物异烟肼的异构体形式. 在评估对甲型/H1N1、A/H3N2 和 B 型流感病毒的活性时,三种新化合物证明对甲型/H3N2 流感病毒具有特异性抗病毒活性。最活跃的类似物3a,在螺环上带有 2,8-二甲基基团,显示出 5.2 µM 的抗病毒 EC 50值。化合物3a在 100 µM(测试的最高浓度)下没有产生细胞毒性,选择性指数至少为 19。构效关系分析表明,在 2 位不存在甲基取代基,而在 8 位存在庞大的取代基。螺噻唑烷酮系统的位置导致抗病毒活性显着降低。化合物2a-f和3a-f的体外抗结核活性对六种不同的药物敏感/耐药实验室菌株和结核分枝杆菌临床分离株进行了测定。化合物2c、2d、3b、3c和3d对不同菌株显示出较弱的抗结核活性,MIC 值为 125-250 μM。






























 京公网安备 11010802027423号
京公网安备 11010802027423号