当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Moving Metal Ions through Ferritin−Protein Nanocages from Three-Fold Pores to Catalytic Sites
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2010-10-20 , DOI: 10.1021/ja105583d Takehiko Tosha 1 , Ho-Leung Ng , Onita Bhattasali , Tom Alber , Elizabeth C Theil
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2010-10-20 , DOI: 10.1021/ja105583d Takehiko Tosha 1 , Ho-Leung Ng , Onita Bhattasali , Tom Alber , Elizabeth C Theil
Affiliation
|
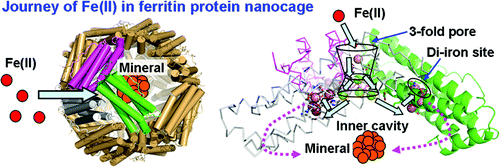
Ferritin nanocages synthesize ferric oxide minerals, containing hundreds to thousands of Fe(III) diferric oxo/hydroxo complexes, by reactions of Fe(II) ions with O(2) at multiple di-iron catalytic centers. Ferric-oxy multimers, tetramers, and/or larger mineral nuclei form during postcatalytic transit through the protein cage, and mineral accretion occurs in the central cavity. We determined how Fe(II) substrates can access catalytic sites using frog M ferritins, active and inactivated by ligand substitution, crystallized with 2.0 M Mg(II) ± 0.1 M Co(II) for Co(II)-selective sites. Co(II) inhibited Fe(II) oxidation. High-resolution (<1.5 Å) crystal structures show (1) a line of metal ions, 15 Å long, which penetrates the cage and defines ion channels and internal pores to the nanocavity that link external pores to the cage interior, (2) metal ions near negatively charged residues at the channel exits and along the inner cavity surface that model Fe(II) transit to active sites, and (3) alternate side-chain conformations, absent in ferritins with catalysis eliminated by amino acid substitution, which support current models of protein dynamics and explain changes in Fe-Fe distances observed during catalysis. The new structural data identify a ∼27-Å path Fe(II) ions can follow through ferritin entry channels between external pores and the central cavity and along the cavity surface to the active sites where mineral synthesis begins. This "bucket brigade" for Fe(II) ion access to the ferritin catalytic sites not only increases understanding of biological nanomineral synthesis but also reveals unexpected design principles for protein cage-based catalysts and nanomaterials.
中文翻译:

将金属离子通过铁蛋白-蛋白质纳米笼从三重孔移动到催化位点
铁蛋白纳米笼通过 Fe(II) 离子与 O(2) 在多个双铁催化中心反应,合成含有数百至数千个 Fe(III) 二铁氧/羟基络合物的氧化铁矿物。在催化后穿过蛋白笼的过程中,铁氧多聚体、四聚体和/或较大的矿物核形成,并且矿物积聚发生在中央空腔中。我们确定了 Fe(II) 底物如何使用青蛙 M 铁蛋白进入催化位点,通过配体取代激活和失活,用 2.0 M Mg(II) ± 0.1 M Co(II) 结晶作为 Co(II) 选择性位点。 Co(II) 抑制 Fe(II) 氧化。高分辨率 (<1.5 Å) 晶体结构显示 (1) 一条 15 Å 长的金属离子线,它穿透笼子并定义离子通道和纳米腔的内部孔,将外部孔与笼子内部连接起来,( 2) 通道出口处的带负电残基附近的金属离子以及沿着内腔表面模拟 Fe(II) 迁移到活性位点的金属离子,以及 (3) 替代侧链构象,铁蛋白中不存在这种构象,通过氨基酸取代消除了催化作用,支持当前的蛋白质动力学模型并解释催化过程中观察到的 Fe-Fe 距离的变化。新的结构数据确定了~27-Å路径,Fe(II)离子可以穿过外部孔隙和中央空腔之间的铁蛋白进入通道,并沿着空腔表面到达矿物合成开始的活性位点。这种铁(II)离子进入铁蛋白催化位点的“桶队”不仅增加了对生物纳米矿物合成的理解,而且揭示了基于蛋白质笼的催化剂和纳米材料的意想不到的设计原理。
更新日期:2010-10-20
中文翻译:

将金属离子通过铁蛋白-蛋白质纳米笼从三重孔移动到催化位点
铁蛋白纳米笼通过 Fe(II) 离子与 O(2) 在多个双铁催化中心反应,合成含有数百至数千个 Fe(III) 二铁氧/羟基络合物的氧化铁矿物。在催化后穿过蛋白笼的过程中,铁氧多聚体、四聚体和/或较大的矿物核形成,并且矿物积聚发生在中央空腔中。我们确定了 Fe(II) 底物如何使用青蛙 M 铁蛋白进入催化位点,通过配体取代激活和失活,用 2.0 M Mg(II) ± 0.1 M Co(II) 结晶作为 Co(II) 选择性位点。 Co(II) 抑制 Fe(II) 氧化。高分辨率 (<1.5 Å) 晶体结构显示 (1) 一条 15 Å 长的金属离子线,它穿透笼子并定义离子通道和纳米腔的内部孔,将外部孔与笼子内部连接起来,( 2) 通道出口处的带负电残基附近的金属离子以及沿着内腔表面模拟 Fe(II) 迁移到活性位点的金属离子,以及 (3) 替代侧链构象,铁蛋白中不存在这种构象,通过氨基酸取代消除了催化作用,支持当前的蛋白质动力学模型并解释催化过程中观察到的 Fe-Fe 距离的变化。新的结构数据确定了~27-Å路径,Fe(II)离子可以穿过外部孔隙和中央空腔之间的铁蛋白进入通道,并沿着空腔表面到达矿物合成开始的活性位点。这种铁(II)离子进入铁蛋白催化位点的“桶队”不仅增加了对生物纳米矿物合成的理解,而且揭示了基于蛋白质笼的催化剂和纳米材料的意想不到的设计原理。































 京公网安备 11010802027423号
京公网安备 11010802027423号