当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding and Modifying the Scaling Relations for Ammonia Synthesis on Dilute Metal Alloys: From Single-Atom Alloys to Dimer Alloys
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-07-15 , DOI: 10.1021/acscatal.2c00745
Yining Zhang 1 , Sha Li 2 , Chao Sun 1 , Ping Wang 2 , Yijun Yang 3 , Ding Yi 3 , Xi Wang 3 , Jiannian Yao 4
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-07-15 , DOI: 10.1021/acscatal.2c00745
Yining Zhang 1 , Sha Li 2 , Chao Sun 1 , Ping Wang 2 , Yijun Yang 3 , Ding Yi 3 , Xi Wang 3 , Jiannian Yao 4
Affiliation
|
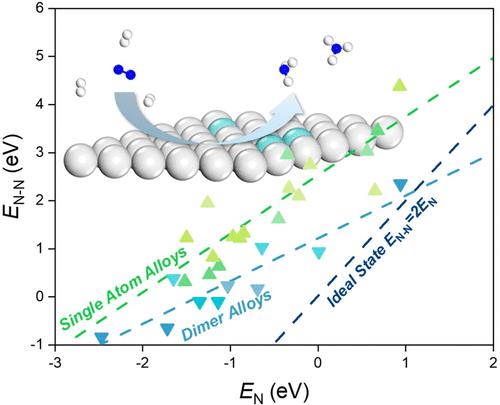
Industrial ammonia synthesis through the Haber–Bosch process operated under harsh reaction conditions leaves ample room for improvement through material design. Designing a catalyst with high activity and low cost is considered as the key to enable large-scale operation under mild conditions. In this work, dilute metal alloys are studied using density functional theory (DFT) calculations and microkinetic modeling to investigate their catalytic performance for ammonia synthesis. Thermochemical scaling relations developed between reaction intermediates and Brønsted–Evans–Polanyi (BEP) relations developed for *N2 dissociation and *NHx hydrogenation form the basis of microkinetic simulations of reaction rates. A degree of rate control analysis shows that the overall reaction is rate-controlled by either *N2 dissociation or *NH2 hydrogenation, resulting in a volcano plot for single-atom alloys (SAAs) with Nb-doped Ag(111) SAA siting at the volcano peak. The BEP relationship for N2 dissociation derived on dimer alloys is closer to the ideal limit in comparison to that obtained on SAAs, leading to higher activities of dimer alloys for ammonia synthesis. Among the dimer alloys, Mo2/Ag(111) is not only more active than the commercial Ru catalysts but also very stable under real reaction conditions and could potentially be used in industrial processes.
中文翻译:

理解和修改稀金属合金合成氨的比例关系:从单原子合金到二聚体合金
通过在恶劣反应条件下运行的 Haber-Bosch 工艺进行工业氨合成,通过材料设计有很大的改进空间。设计具有高活性和低成本的催化剂被认为是在温和条件下实现大规模操作的关键。在这项工作中,使用密度泛函理论 (DFT) 计算和微动力学建模研究了稀金属合金,以研究它们对氨合成的催化性能。反应中间体之间发展的热化学标度关系和针对 *N 2解离和 *NH x发展的 Brønsted-Evans-Polanyi (BEP) 关系加氢形成反应速率微动力学模拟的基础。一定程度的速率控制分析表明,整个反应由 *N 2解离或 *NH 2加氢来控制,导致具有 Nb 掺杂 Ag(111) SAA 位点的单原子合金 (SAA) 的火山图在火山峰。与在 SAA 上获得的相比,在二聚体合金上获得的 N 2解离的 BEP 关系更接近理想极限,从而导致二聚体合金的氨合成活性更高。在二聚体合金中,Mo 2 /Ag(111) 不仅比市售的 Ru 催化剂活性更高,而且在实际反应条件下也非常稳定,有可能用于工业过程。
更新日期:2022-07-15
中文翻译:

理解和修改稀金属合金合成氨的比例关系:从单原子合金到二聚体合金
通过在恶劣反应条件下运行的 Haber-Bosch 工艺进行工业氨合成,通过材料设计有很大的改进空间。设计具有高活性和低成本的催化剂被认为是在温和条件下实现大规模操作的关键。在这项工作中,使用密度泛函理论 (DFT) 计算和微动力学建模研究了稀金属合金,以研究它们对氨合成的催化性能。反应中间体之间发展的热化学标度关系和针对 *N 2解离和 *NH x发展的 Brønsted-Evans-Polanyi (BEP) 关系加氢形成反应速率微动力学模拟的基础。一定程度的速率控制分析表明,整个反应由 *N 2解离或 *NH 2加氢来控制,导致具有 Nb 掺杂 Ag(111) SAA 位点的单原子合金 (SAA) 的火山图在火山峰。与在 SAA 上获得的相比,在二聚体合金上获得的 N 2解离的 BEP 关系更接近理想极限,从而导致二聚体合金的氨合成活性更高。在二聚体合金中,Mo 2 /Ag(111) 不仅比市售的 Ru 催化剂活性更高,而且在实际反应条件下也非常稳定,有可能用于工业过程。

































 京公网安备 11010802027423号
京公网安备 11010802027423号