当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
tert-Butyl Hydroperoxide Promoted the Reaction of Quinazoline-3-oxides with Primary Amines Affording Quinazolin-4(3H)-ones
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-07-14 , DOI: 10.1021/acs.joc.2c00898 Jin Luo 1 , Juelin Wan 2 , Lianlian Wu 2 , Lingyun Yang 2 , Tao Wang 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-07-14 , DOI: 10.1021/acs.joc.2c00898 Jin Luo 1 , Juelin Wan 2 , Lianlian Wu 2 , Lingyun Yang 2 , Tao Wang 2
Affiliation
|
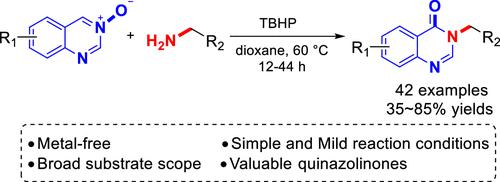
An efficient and facile approach for the synthesis of quinazolin-4(3H)-ones via the reaction of quinazoline-3-oxides with primary amines is described. This approach is demonstrated to be applicable for a broad range of substrates and proceeds efficiently under metal-free and mild reaction conditions employing easily available tert-butyl hydroperoxide as the oxidant. Remarkably, 3-(2-(1H-indol-3-yl) ethyl)quinazolin-4(3H)-one 3w, which was conveniently obtained by this process in 70% yield, was an excellent precursor for the synthesis of bioactive evodiamine and rutaempine.
中文翻译:

叔丁基过氧化氢促进喹唑啉-3-氧化物与提供Quinazolin-4(3H)-ones的伯胺反应
介绍了一种通过 quinazolin-3-氧化物与伯胺反应合成 quinazolin-4(3 H )-ones 的有效且简便的方法。这种方法被证明适用于广泛的底物,并且在使用容易获得的叔丁基过氧化氢作为氧化剂的无金属和温和的反应条件下有效地进行。值得注意的是,3-(2-(1 H -indol-3-yl) ethyl)quinazolin-4(3 H )-one 3w是通过该方法方便地以 70% 的收率获得的,是合成生物活性吴茱萸碱和鲁坦平。
更新日期:2022-07-14
中文翻译:

叔丁基过氧化氢促进喹唑啉-3-氧化物与提供Quinazolin-4(3H)-ones的伯胺反应
介绍了一种通过 quinazolin-3-氧化物与伯胺反应合成 quinazolin-4(3 H )-ones 的有效且简便的方法。这种方法被证明适用于广泛的底物,并且在使用容易获得的叔丁基过氧化氢作为氧化剂的无金属和温和的反应条件下有效地进行。值得注意的是,3-(2-(1 H -indol-3-yl) ethyl)quinazolin-4(3 H )-one 3w是通过该方法方便地以 70% 的收率获得的,是合成生物活性吴茱萸碱和鲁坦平。







































 京公网安备 11010802027423号
京公网安备 11010802027423号