当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A stable single-atom Zn catalyst synthesized by a ligand-stabilized pyrolysis strategy for selective oxidation of C–H bonds
Green Chemistry ( IF 9.3 ) Pub Date : 2022-07-14 , DOI: 10.1039/d2gc01831d
Wenhui Wang 1 , Ningzhao Shang 1 , Junmin Wang 1 , Xinhao Nie 1 , Congcong Du 2 , Xin Zhou 1 , Xiang Cheng 1 , Wei Gao 1 , Xue Liu 1 , Jianyu Huang 2 , Yuqing Qiao 2 , Shutao Gao 1 , Chun Wang 1
Green Chemistry ( IF 9.3 ) Pub Date : 2022-07-14 , DOI: 10.1039/d2gc01831d
Wenhui Wang 1 , Ningzhao Shang 1 , Junmin Wang 1 , Xinhao Nie 1 , Congcong Du 2 , Xin Zhou 1 , Xiang Cheng 1 , Wei Gao 1 , Xue Liu 1 , Jianyu Huang 2 , Yuqing Qiao 2 , Shutao Gao 1 , Chun Wang 1
Affiliation
|
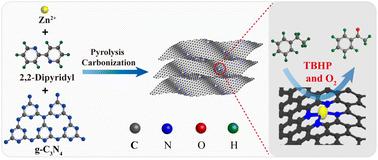
The activation of C–H bonds for the synthesis of highly valued chemicals is still a challenging task. Herein, a nitrogen-doped carbon supported single atom Zn catalyst (Zn–N–C) was fabricated via a ligand-stabilized pyrolysis strategy, in which 2,2′-bipyridine (bipy) served as a ligand and graphitic carbon nitride (g-C3N4) served as a directional 2D-template and a nitrogen source. The results of high-angle annular dark field aberration corrected scanning transmission electron microscopy (HAADF-STEM) and extended X-ray absorption fine structure (EXAFS) spectroscopy indicated that Zn species are present as isolated single sites and stabilized by nitrogen in the Zn–N4 structure. The optimized Zn–N–C-900 catalyst displayed superior catalytic performance for the selective oxidation of C–H bonds. In particular, the Zn–N–C-900 catalyst can be reused fifteen times without obvious decay in either activity or selectivity. Density functional theory calculations were conducted to reveal the reaction mechanism. The results indicated that the high valence Zn![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species generated on Zn–N–C-900 were the crucial active centers for the activation of C–H bonds.
O species generated on Zn–N–C-900 were the crucial active centers for the activation of C–H bonds.
中文翻译:

通过配体稳定的热解策略合成稳定的单原子 Zn 催化剂,用于 C-H 键的选择性氧化
用于合成高价值化学品的 C-H 键活化仍然是一项具有挑战性的任务。在此,通过配体稳定的热解策略制备了一种氮掺杂的碳负载单原子 Zn 催化剂 (Zn-N-C) ,其中 2,2'-联吡啶 (bipy) 作为配体和石墨氮化碳 (gC) 3 N 4 ) 用作定向二维模板和氮源。高角度环形暗场像差校正扫描透射电子显微镜 (HAADF-STEM) 和扩展 X 射线吸收精细结构 (EXAFS) 光谱的结果表明,Zn 物种以孤立的单点形式存在,并被 Zn–氮4结构体。优化后的 Zn-N-C-900 催化剂对 C-H 键的选择性氧化表现出优异的催化性能。特别是 Zn-N-C-900 催化剂可以重复使用 15 次,而活性或选择性都没有明显下降。进行密度泛函理论计算以揭示反应机理。结果表明,在 Zn-N-C-900 上产生的高价 ZnO![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) 物种是激活 C-H 键的关键活性中心。
物种是激活 C-H 键的关键活性中心。
更新日期:2022-07-14
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species generated on Zn–N–C-900 were the crucial active centers for the activation of C–H bonds.
O species generated on Zn–N–C-900 were the crucial active centers for the activation of C–H bonds.
中文翻译:

通过配体稳定的热解策略合成稳定的单原子 Zn 催化剂,用于 C-H 键的选择性氧化
用于合成高价值化学品的 C-H 键活化仍然是一项具有挑战性的任务。在此,通过配体稳定的热解策略制备了一种氮掺杂的碳负载单原子 Zn 催化剂 (Zn-N-C) ,其中 2,2'-联吡啶 (bipy) 作为配体和石墨氮化碳 (gC) 3 N 4 ) 用作定向二维模板和氮源。高角度环形暗场像差校正扫描透射电子显微镜 (HAADF-STEM) 和扩展 X 射线吸收精细结构 (EXAFS) 光谱的结果表明,Zn 物种以孤立的单点形式存在,并被 Zn–氮4结构体。优化后的 Zn-N-C-900 催化剂对 C-H 键的选择性氧化表现出优异的催化性能。特别是 Zn-N-C-900 催化剂可以重复使用 15 次,而活性或选择性都没有明显下降。进行密度泛函理论计算以揭示反应机理。结果表明,在 Zn-N-C-900 上产生的高价 ZnO
![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) 物种是激活 C-H 键的关键活性中心。
物种是激活 C-H 键的关键活性中心。

































 京公网安备 11010802027423号
京公网安备 11010802027423号