当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metal-Free Temperature-Controlled Intermolecular [3 + 2] Annulation to Access Benzo[d]thiazole-2(3H)-thiones and Benzo[d]thiazol-2(3H)-ones
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-07-12 , DOI: 10.1021/acs.joc.2c01060 Xiaoya Zhuo 1 , Lvyin Zheng 1 , Xiaoying Zou 1 , Yumei Zhong 1 , Wei Guo 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-07-12 , DOI: 10.1021/acs.joc.2c01060 Xiaoya Zhuo 1 , Lvyin Zheng 1 , Xiaoying Zou 1 , Yumei Zhong 1 , Wei Guo 1
Affiliation
|
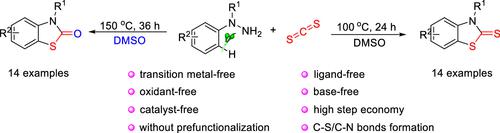
A facile access to benzo[d]thiazole-2(3H)-thiones and benzo[d]thiazol-2(3H)-ones has been developed through a temperature-controlled intermolecular [3 + 2] annulation of N,N-disubstituted arylhydrazines with CS2 in the presence of DMSO. This protocol can obviate the prefunctionalization of the starting materials. This direct C–S/C–N bond formation reaction was performed in the absence of any external catalysts, transition metals, bases, ligands, and oxidants with high step economy.
中文翻译:

无金属温控分子间 [3 + 2] 环化以获取苯并[d]噻唑-2(3H)-硫酮和苯并[d]噻唑-2(3H)-酮
通过温度控制的 N,N 分子间 [3 + 2] 环化,开发了一种容易获得苯并[ d ]噻唑-2(3 H )-硫酮和苯并[ d ]噻唑-2(3 H )-酮的方法-在DMSO存在下用CS 2二取代的芳基肼。该协议可以避免起始材料的预功能化。这种直接的 C-S/C-N 键形成反应是在没有任何外部催化剂、过渡金属、碱、配体和氧化剂的情况下进行的,具有高步骤经济性。
更新日期:2022-07-12
中文翻译:

无金属温控分子间 [3 + 2] 环化以获取苯并[d]噻唑-2(3H)-硫酮和苯并[d]噻唑-2(3H)-酮
通过温度控制的 N,N 分子间 [3 + 2] 环化,开发了一种容易获得苯并[ d ]噻唑-2(3 H )-硫酮和苯并[ d ]噻唑-2(3 H )-酮的方法-在DMSO存在下用CS 2二取代的芳基肼。该协议可以避免起始材料的预功能化。这种直接的 C-S/C-N 键形成反应是在没有任何外部催化剂、过渡金属、碱、配体和氧化剂的情况下进行的,具有高步骤经济性。































 京公网安备 11010802027423号
京公网安备 11010802027423号