当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure and Valency of a Cobalt−Phosphate Water Oxidation Catalyst Determined by in Situ X-ray Spectroscopy
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2010-10-06 , DOI: 10.1021/ja1023767 Matthew W. Kanan 1 , Junko Yano 1 , Yogesh Surendranath 1 , Mircea Dincă 1 , Vittal K. Yachandra 1 , Daniel G. Nocera 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2010-10-06 , DOI: 10.1021/ja1023767 Matthew W. Kanan 1 , Junko Yano 1 , Yogesh Surendranath 1 , Mircea Dincă 1 , Vittal K. Yachandra 1 , Daniel G. Nocera 1
Affiliation
|
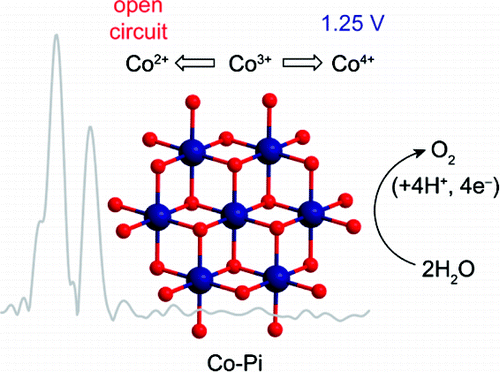
A water oxidation catalyst generated via electrodeposition from aqueous solutions containing phosphate and Co(2+) (Co-Pi) has been studied by in situ X-ray absorption spectroscopy. Spectra were obtained for Co-Pi films of two different thicknesses at an applied potential supporting water oxidation catalysis and at open circuit. Extended X-ray absorption fine structure (EXAFS) spectra indicate the presence of bis-oxo/hydroxo-bridged Co subunits incorporated into higher nuclearity clusters in Co-Pi. The average cluster nuclearity is greater in a relatively thick film (∼40-50 nmol Co ions/cm(2)) deposited at 1.25 V vs NHE than in an extremely thin film (∼3 nmol Co ions/cm(2)) deposited at 1.1 V. X-ray absorption near edge structure (XANES) spectra and electrochemical data support a Co valency greater than 3 for both Co-Pi samples when catalyzing water oxidation at 1.25 V. Upon switching to open circuit, Co-Pi undergoes a continuous reduction due to residual water oxidation catalysis, as indicated by the negative shift of the edge energy. The rate of reduction depends on the average cluster size. On the basis of structural parameters extracted from fits to the EXAFS data of Co-Pi with two different thicknesses and comparisons with EXAFS spectra of Co oxide compounds, a model is proposed wherein the Co oxo/hydroxo clusters of Co-Pi are composed of edge-sharing CoO(6) octahedra, the structural motif found in cobaltates. Whereas cobaltates contain extended planes of CoO(6) octahedra, the Co-Pi clusters are of molecular dimensions.
中文翻译:

原位 X 射线光谱法测定钴-磷酸盐水氧化催化剂的结构和价态
通过原位 X 射线吸收光谱法研究了通过电沉积从含有磷酸盐和 Co(2+) (Co-Pi) 的水溶液中生成的水氧化催化剂。在支持水氧化催化和开路的外加电位下,获得了两种不同厚度的 Co-Pi 薄膜的光谱。扩展的 X 射线吸收精细结构 (EXAFS) 光谱表明存在双氧/羟基桥接的 Co 亚基,这些 Co 亚基并入 Co-Pi 中更高的核度簇。在相对于 NHE 的 1.25 V 电压下沉积的相对较厚的薄膜(~40-50 nmol Co 离子/cm(2))中的平均簇核比沉积在极薄的薄膜中(~3 nmol Co 离子/cm(2))更大在 1.1 V。X 射线吸收近边结构 (XANES) 光谱和电化学数据支持两种 Co-Pi 样品在 1.25 V 下催化水氧化时的 Co 价大于 3。在切换到开路时,Co-Pi 由于以下原因持续还原残余水氧化催化,如边缘能量的负移所示。减少率取决于平均簇大小。基于对两种不同厚度的 Co-Pi 的 EXAFS 数据拟合提取的结构参数,并与 Co 氧化物的 EXAFS 光谱进行比较,提出了一个模型,其中 Co-Pi 的 Co 氧/羟基簇由边缘组成。 -共享 CoO(6) 八面体,在钴酸盐中发现的结构基序。虽然钴酸盐包含 CoO(6) 八面体的扩展平面,但 Co-Pi 簇具有分子尺寸。
更新日期:2010-10-06
中文翻译:

原位 X 射线光谱法测定钴-磷酸盐水氧化催化剂的结构和价态
通过原位 X 射线吸收光谱法研究了通过电沉积从含有磷酸盐和 Co(2+) (Co-Pi) 的水溶液中生成的水氧化催化剂。在支持水氧化催化和开路的外加电位下,获得了两种不同厚度的 Co-Pi 薄膜的光谱。扩展的 X 射线吸收精细结构 (EXAFS) 光谱表明存在双氧/羟基桥接的 Co 亚基,这些 Co 亚基并入 Co-Pi 中更高的核度簇。在相对于 NHE 的 1.25 V 电压下沉积的相对较厚的薄膜(~40-50 nmol Co 离子/cm(2))中的平均簇核比沉积在极薄的薄膜中(~3 nmol Co 离子/cm(2))更大在 1.1 V。X 射线吸收近边结构 (XANES) 光谱和电化学数据支持两种 Co-Pi 样品在 1.25 V 下催化水氧化时的 Co 价大于 3。在切换到开路时,Co-Pi 由于以下原因持续还原残余水氧化催化,如边缘能量的负移所示。减少率取决于平均簇大小。基于对两种不同厚度的 Co-Pi 的 EXAFS 数据拟合提取的结构参数,并与 Co 氧化物的 EXAFS 光谱进行比较,提出了一个模型,其中 Co-Pi 的 Co 氧/羟基簇由边缘组成。 -共享 CoO(6) 八面体,在钴酸盐中发现的结构基序。虽然钴酸盐包含 CoO(6) 八面体的扩展平面,但 Co-Pi 簇具有分子尺寸。






























 京公网安备 11010802027423号
京公网安备 11010802027423号