当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preparation of Maleimide-Modified Oligonucleotides from the Corresponding Amines Using N-Methoxycarbonylmaleimide
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2022-07-11 , DOI: 10.1021/acs.bioconjchem.2c00144 Nanna L Kjærsgaard 1, 2 , Rikke A Hansen 1, 2 , Kurt V Gothelf 1, 2
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2022-07-11 , DOI: 10.1021/acs.bioconjchem.2c00144 Nanna L Kjærsgaard 1, 2 , Rikke A Hansen 1, 2 , Kurt V Gothelf 1, 2
Affiliation
|
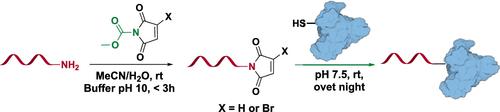
Oligonucleotide conjugates constitute a versatile tool for research and bioanalytical purposes. Often, such conjugates are prepared by reaction between a thiol on the protein with a maleimide-modified oligonucleotide. Unlike most other chemical handles the maleimide functionality cannot be introduced directly during the solid-phase oligonucleotide synthesis, and therefore the standard method to introduce the maleimide functionality is to react an amino-modified DNA with a heterobifunctional linker containing an activated ester and a maleimide. Here, we present an alternative method for preparation of maleimide and monobromomaleimide-modified oligonucleotides from the corresponding amine using N-methoxycarbonylmaleimide and N-methoxycarbonylbromomaleimide, respectively. In this method, no additional linker is attached to the oligonucleotide, as the maleimide functionality is formed directly on the existing amine. The maleimide can thereby be positioned close to the oligonucleotide, providing a high degree of control over the final construct. The reaction occurs in 30–60 min under alkaline conditions. Maleimide-modified oligonucleotides prepared in this manner were conjugated to bovine serum albumin, and the reaction shows comparable reactivity to the corresponding oligonucleotide modified using the 4-(N-maleimidomethyl)-cyclohexane-1-carboxylate (SMCC) linker.
中文翻译:

使用 N-甲氧基羰基马来酰亚胺从相应的胺制备马来酰亚胺修饰的寡核苷酸
寡核苷酸偶联物构成了用于研究和生物分析目的的多功能工具。通常,此类缀合物通过蛋白质上的硫醇与马来酰亚胺修饰的寡核苷酸之间的反应来制备。与大多数其他化学处理不同,马来酰亚胺官能团不能在固相寡核苷酸合成过程中直接引入,因此引入马来酰亚胺官能团的标准方法是使氨基修饰的 DNA 与含有活化酯和马来酰亚胺的异双功能接头反应。在这里,我们提出了一种使用N-甲氧基羰基马来酰亚胺和N从相应胺制备马来酰亚胺和单溴马来酰亚胺修饰的寡核苷酸的替代方法-甲氧基羰基溴马来酰亚胺,分别。在该方法中,没有额外的接头连接到寡核苷酸上,因为马来酰亚胺官能团直接在现有胺上形成。马来酰亚胺因此可以靠近寡核苷酸定位,提供对最终构建体的高度控制。在碱性条件下,反应在 30-60 分钟内发生。以这种方式制备的马来酰亚胺修饰的寡核苷酸与牛血清白蛋白结合,该反应显示出与使用 4-( N-马来酰亚胺甲基)-环己烷-1-羧酸酯 (SMCC) 接头修饰的相应寡核苷酸相当的反应性。
更新日期:2022-07-11
中文翻译:

使用 N-甲氧基羰基马来酰亚胺从相应的胺制备马来酰亚胺修饰的寡核苷酸
寡核苷酸偶联物构成了用于研究和生物分析目的的多功能工具。通常,此类缀合物通过蛋白质上的硫醇与马来酰亚胺修饰的寡核苷酸之间的反应来制备。与大多数其他化学处理不同,马来酰亚胺官能团不能在固相寡核苷酸合成过程中直接引入,因此引入马来酰亚胺官能团的标准方法是使氨基修饰的 DNA 与含有活化酯和马来酰亚胺的异双功能接头反应。在这里,我们提出了一种使用N-甲氧基羰基马来酰亚胺和N从相应胺制备马来酰亚胺和单溴马来酰亚胺修饰的寡核苷酸的替代方法-甲氧基羰基溴马来酰亚胺,分别。在该方法中,没有额外的接头连接到寡核苷酸上,因为马来酰亚胺官能团直接在现有胺上形成。马来酰亚胺因此可以靠近寡核苷酸定位,提供对最终构建体的高度控制。在碱性条件下,反应在 30-60 分钟内发生。以这种方式制备的马来酰亚胺修饰的寡核苷酸与牛血清白蛋白结合,该反应显示出与使用 4-( N-马来酰亚胺甲基)-环己烷-1-羧酸酯 (SMCC) 接头修饰的相应寡核苷酸相当的反应性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号