Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2022-07-08 , DOI: 10.1016/j.jhazmat.2022.129552 Zhenchao Lei 1 , Ziyuan Huang 1 , Yimin Lin 1 , Yuwei Liu 1 , Zhang Yan 1 , Wenxiao Zheng 1 , Huanxin Ma 1 , Zhi Dang 1 , Chunhua Feng 1
|
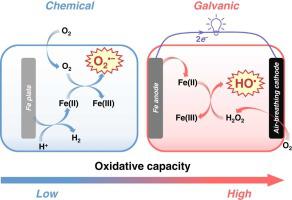
The corrosion of Fe(0) in the presence of O2 in nature can lead to the oxidation of organic compounds, but the efficiency is very limited. Herein, attempts were made to establish a galvanic system that separates the anodic Fe(0) oxidation reaction and the cathodic O2 reduction reaction using an air-breathing cathode. Compared with the chemical Fe(0)/O2 system, it exhibited a substantially higher capability of destroying a variety of pollutants, such as organic dyes (12 types), phenol, nitrobenzene, acetaminophen, phenol, and ethylenediaminetetraacetic acid. The degradation rate constant of a model dye (i.e., Rhodamine B) increased from 0.047 min−1 (chemical) to 1.412 min−1 (galvanic) under the passive air-breathing condition. The electric circuit design promoted Fe(0) dissolution to Fe(II) and triggered electron transfer that drives O2 reduction to H2O2, two important species responsible for the generation of HO• at high abundance. In addition, the galvanic Fe(0)/O2 system produces electricity while destroying pollutants. Tests with real Ni plating wastewater further demonstrated the capability of the system to oxidize complexed organics and phosphite. This study provides a new strategy for boosting the oxidative capacity of the Fe(0)/O2 system, which shows promise for acid wastewater treatment.
中文翻译:

通过吸气阴极提高 Fe(0)/O2 系统的氧化能力
Fe(0)在自然界中O 2存在下的腐蚀会导致有机化合物的氧化,但效率非常有限。在此,尝试建立使用吸气式阴极分离阳极Fe(0)氧化反应和阴极O 2还原反应的原电池系统。与化学Fe(0)/O 2体系相比,它对有机染料(12种)、苯酚、硝基苯、对乙酰氨基酚、苯酚、乙二胺四乙酸等多种污染物的破坏能力显着提高。模型染料(即罗丹明 B)的降解速率常数从 0.047 min -1(化学)增加到 1.412 min -1(电流)在被动吸气条件下。电路设计促进了 Fe(0) 溶解为 Fe(II) 并触发了电子转移,从而驱动 O 2还原为 H 2 O 2,这两种重要物质负责以高丰度生成 HO • • 。此外,原电池 Fe(0)/O 2系统可在发电的同时破坏污染物。用真正的镍电镀废水进行的测试进一步证明了该系统氧化络合有机物和亚磷酸盐的能力。本研究为提高 Fe(0)/O 2系统的氧化能力提供了一种新策略,这显示了酸性废水处理的前景。






























 京公网安备 11010802027423号
京公网安备 11010802027423号