Synlett ( IF 1.7 ) Pub Date : 2022-07-08 , DOI: 10.1055/a-1863-8957 Baixue Luan 1 , Zaiquan Tang 1 , Xianqing Wu 1 , Yifeng Chen 1
|
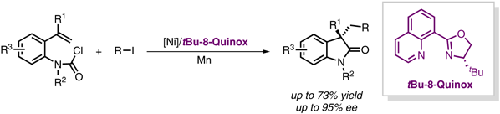
Chiral ligands play an essential role in transition-metal-catalyzed enantioselective transformations, in which chiral oxazoline-based scaffolds are the privileged chiral ligand. Nevertheless, 8-quinolinyl oxazoline (8-Quinox) ligands are underexplored in transition-metal-catalyzed asymmetric transformations since their development in 1998. Herein, we report an 8-Quinox ligand promoted Ni-catalyzed enantioselective reductive carbamoyl-alkylation of carbamoyl chloride tethered styrene with unactivated alkyl iodide, providing an expedient access to valuable enantioenriched oxindoles in good results.
中文翻译:

8-喹啉基恶唑啉:对映选择性镍催化还原氨甲酰烷基化烯烃获得手性恶唑啉的配体探索
手性配体在过渡金属催化的对映选择性转化中发挥着重要作用,其中手性恶唑啉基支架是优先的手性配体。然而,自 1998 年开发以来,8-喹啉基恶唑啉 (8-Quinox) 配体在过渡金属催化的不对称转化中的探索不足。在此,我们报告了一种 8-Quinox 配体促进了 Ni 催化的对映选择性还原氨基甲酰烷基化的氨基甲酰氯苯乙烯与未活化的烷基碘,为获得有价值的对映体富集的羟吲哚提供了便利,并取得了良好的效果。































 京公网安备 11010802027423号
京公网安备 11010802027423号