Chem Catalysis ( IF 11.5 ) Pub Date : 2022-07-08 , DOI: 10.1016/j.checat.2022.06.005 Suchen Zou , Bangkui Yu , Hanmin Huang
|
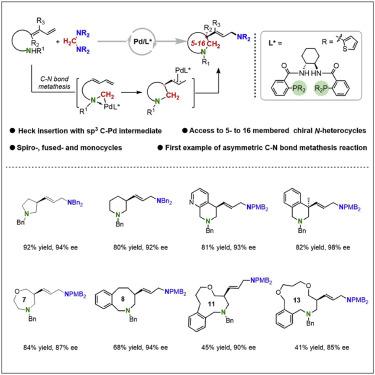
Optically active N-heterocycles are important building blocks in organic synthesis and medicinal chemistry. However, an enantioselective synthetic protocol with excellent compatibility across the normal-sized, medium-sized, and macro-sized rings remains largely rare. We herein report a Pd-catalyzed highly enantioselective ring-closing aminomethylamination of aminodienes with aminals via C–N bond metathesis, which provides rapid access to enantiomer-enriched 5- to 16-membered β-aminoallylated N-heterocycles (up to 98% ee) under mild conditions with the use of modified Trost ligands. In addition, the enantioselective synthesis of chiral tetrahydroisoquinolines (THIQs) has also been realized by this method. The unity of this method has been demonstrated by the formal synthesis of the natural product (+)-(8S,13R)-cyclocelabenzine. Mechanistically, the reaction goes through a challenging and less known stereoselective migratory insertion of alkene into an alkyl-Pd species. We also conducted a detailed analysis of the stereochemical outcome of the reaction, which provided an intriguing view of enantioselection.
中文翻译:

通过修饰的Trost配体实现氨基二烯的对映选择性闭环氨基甲基胺化
光学活性N-杂环是有机合成和药物化学中的重要组成部分。然而,在正常尺寸、中型和宏观尺寸环之间具有出色兼容性的对映选择性合成方案仍然很少见。我们在此报告了 Pd 催化的氨基二烯与缩醛胺通过 C-N 键复分解的高对映选择性闭环氨基甲基胺化,这提供了快速获得富含对映异构体的 5 至 16 元 β-氨基烯丙基化N-杂环(高达 98% ee)在温和条件下使用修饰的 Trost 配体。此外,该方法还实现了手性四氢异喹啉(THIQs)的对映选择性合成。天然产物 (+)-( 8S , 13R )-cyclocelabenzine的正式合成证明了该方法的统一性。从机理上讲,该反应经历了具有挑战性且鲜为人知的将烯烃立体选择性迁移插入到烷基-Pd 物质中的过程。我们还对反应的立体化学结果进行了详细分析,这提供了对映体选择的有趣观点。

































 京公网安备 11010802027423号
京公网安备 11010802027423号