当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaction of α-Diazopyrroles with Enamines: Synthesis of Pyrrolo[2,1-c][1,2,4]triazines and α-(1,2,5-Triazapenta-1,3-dienyl)pyrroles
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-07-08 , DOI: 10.1021/acs.joc.2c01102 Nikita A Kaminskiy 1 , Ekaterina E Galenko 1 , Mariya A Kryukova 1 , Mikhail S Novikov 1 , Alexander F Khlebnikov 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-07-08 , DOI: 10.1021/acs.joc.2c01102 Nikita A Kaminskiy 1 , Ekaterina E Galenko 1 , Mariya A Kryukova 1 , Mikhail S Novikov 1 , Alexander F Khlebnikov 1
Affiliation
|
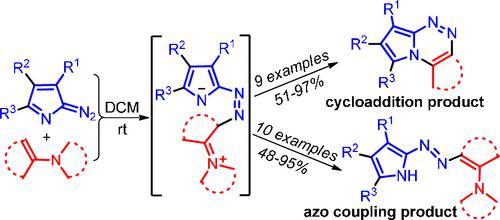
α-Diazopyrroles selectively react with enamines at room temperature to give either (4+2)-cycloaddition–dehydroamination cascade products, pyrrolo[2,1-c][1,2,4]triazines, or azo coupling products. The reaction was used for the synthesis of functionalized ortho-fused heterocycles with new tetrahydrobenzo[e]pyrrolo[2,1-c][1,2,4]triazine and tetrahydro-6H-cyclohepta[e]pyrrolo[2,1-c][1,2,4]triazine frameworks. Unstable azo compounds, 2-[(2-aminovinyl)diazenyl]pyrroles, were obtained from enamines of tetralone or acyclic ketones. According to the density functional theory calculations, (4+2)-cycloaddition of α-diazopyrroles with enamines proceeds in a nonconcerted manner via the zwitterionic intermediate, which undergoes cyclization to pyrrolotriazine or a competitive 1,5-prototropic shift leading to the formation of azo coupling product.
中文翻译:

α-重氮吡咯与烯胺的反应:吡咯并[2,1-c][1,2,4]三嗪和α-(1,2,5-Triazapenta-1,3-二烯基)吡咯的合成
α-重氮吡咯在室温下选择性地与烯胺反应生成 (4+2)-环加成-脱氢胺级联产物、吡咯并[2,1- c ][1,2,4]三嗪或偶氮偶联产物。该反应用于与新的四氢苯并[ e ]吡咯并[2,1- c ][1,2,4]三嗪和四氢-6H-环庚[ e ]吡咯并[2,1]合成官能化的邻位稠合杂环-c _][1,2,4]三嗪框架。从四氢萘酮或无环酮的烯胺中获得不稳定的偶氮化合物,2-[(2-氨基乙烯基)二氮烯基]吡咯。根据密度泛函理论计算,α-重氮吡咯与烯胺的 (4+2)-环加成通过两性离子中间体以非协调方式进行,中间体环化为吡咯并三嗪或竞争性 1,5-质子转变导致形成偶氮偶联产物。
更新日期:2022-07-08
中文翻译:

α-重氮吡咯与烯胺的反应:吡咯并[2,1-c][1,2,4]三嗪和α-(1,2,5-Triazapenta-1,3-二烯基)吡咯的合成
α-重氮吡咯在室温下选择性地与烯胺反应生成 (4+2)-环加成-脱氢胺级联产物、吡咯并[2,1- c ][1,2,4]三嗪或偶氮偶联产物。该反应用于与新的四氢苯并[ e ]吡咯并[2,1- c ][1,2,4]三嗪和四氢-6H-环庚[ e ]吡咯并[2,1]合成官能化的邻位稠合杂环-c _][1,2,4]三嗪框架。从四氢萘酮或无环酮的烯胺中获得不稳定的偶氮化合物,2-[(2-氨基乙烯基)二氮烯基]吡咯。根据密度泛函理论计算,α-重氮吡咯与烯胺的 (4+2)-环加成通过两性离子中间体以非协调方式进行,中间体环化为吡咯并三嗪或竞争性 1,5-质子转变导致形成偶氮偶联产物。































 京公网安备 11010802027423号
京公网安备 11010802027423号