当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper- and Chiral Nitroxide-Catalyzed Oxidative Kinetic Resolution of Axially Chiral N-Arylpyrroles
Organic Letters ( IF 4.9 ) Pub Date : 2022-07-07 , DOI: 10.1021/acs.orglett.2c01860 Lenin Kumar Verdhi 1 , Natalia Fridman 2 , Alex M Szpilman 1
Organic Letters ( IF 4.9 ) Pub Date : 2022-07-07 , DOI: 10.1021/acs.orglett.2c01860 Lenin Kumar Verdhi 1 , Natalia Fridman 2 , Alex M Szpilman 1
Affiliation
|
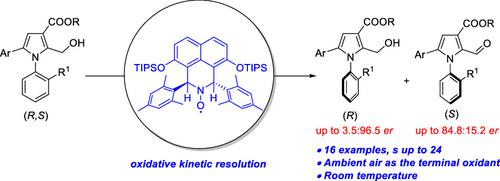
A readily prepared C2-symmetric, α-hydrogen-substituted chiral hydroxylamine serves as a precatalyst to generate a chiral nitroxide in situ. This chiral nitroxide catalyst in combination with a copper co-catalyst functions as an oxidant for an unprecedented enantioselective oxidative kinetic resolution (OKR) of racemic axially chiral N-arylpyrrole alcohols using atmospheric oxygen as an environmentally friendly terminal oxidant. The OKR process provides the axially chiral N-arylpyrroles in er up to 3.5:96.5 and with s factors up to 24.
中文翻译:

铜和手性氮氧化物催化的轴向手性 N-芳基吡咯的氧化动力学拆分
容易制备的 C 2 -对称、α-氢取代的手性羟胺用作原位生成手性氮氧化物的预催化剂。这种手性氮氧化物催化剂与铜助催化剂结合用作氧化剂,使用大气氧作为环境友好的末端氧化剂,对外消旋轴向手性N-芳基吡咯醇进行前所未有的对映选择性氧化动力学拆分 (OKR) 。OKR 工艺提供了高达 3.5:96.5的轴向手性N-芳基吡咯和高达 24的 s因子。
更新日期:2022-07-07
中文翻译:

铜和手性氮氧化物催化的轴向手性 N-芳基吡咯的氧化动力学拆分
容易制备的 C 2 -对称、α-氢取代的手性羟胺用作原位生成手性氮氧化物的预催化剂。这种手性氮氧化物催化剂与铜助催化剂结合用作氧化剂,使用大气氧作为环境友好的末端氧化剂,对外消旋轴向手性N-芳基吡咯醇进行前所未有的对映选择性氧化动力学拆分 (OKR) 。OKR 工艺提供了高达 3.5:96.5的轴向手性N-芳基吡咯和高达 24的 s因子。































 京公网安备 11010802027423号
京公网安备 11010802027423号