Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2022-07-03 , DOI: 10.1016/j.jfluchem.2022.110015 Sagar R Mudshinge 1 , Gerald B Hammond 1 , Teruo Umemoto 1
|
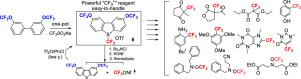
A new, powerful, and easy-to-handle electrophilic trifluoromethylating agent, S-(trifluoromethyl)-2,8-bis(trifluoromethoxy)dibenzothiophenium triflate (Umemoto reagent IV), was developed. Due to the extraordinary electronic effect of trifluoromethoxy group, Umemoto reagent IV was easily synthesized by a one-pot method from readily available 3,3’-bis(trifluoromethoxy)biphenyl. It was shown that Umemoto reagent IV was more powerful than Umemoto reagent II and could trifluoromethylate many kinds of nucleophilic substrates more effectively. In addition, Umemoto reagent IV was successfully utilized for the preparation of trifluoromethyl nonaflate, a useful trifluoromethoxylating agent. The direct conversion of 2,8-bis(trifluoromethoxy)dibenzothiophene to Umemoto reagent IV with triflic anhydride was achieved, albeit in low yield.
中文翻译:

S-(三氟甲基)-2,8-双(三氟甲氧基)二苯并噻吩鎓三氟甲磺酸酯(Umemoto试剂IV)的合成及应用
开发了一种新型、强效且易于操作的亲电三氟甲基化试剂,S- (三氟甲基)-2,8-双(三氟甲氧基)二苯并噻吩鎓三氟甲磺酸盐(Umemoto试剂IV)。由于三氟甲氧基的非凡电子效应,Umemoto试剂IV可以通过易得的3,3'-双(三氟甲氧基)联苯通过一锅法轻松合成。结果表明,Umemoto 试剂 IV 比 Umemoto 试剂 II 更强大,可以更有效地对多种亲核底物进行三氟甲基化。此外,Umemoto试剂IV成功地用于制备有用的三氟甲氧基化剂三氟甲基九氟磺酸酯。实现了用三氟甲磺酸酐将 2,8-双(三氟甲氧基)二苯并噻吩直接转化为 Umemoto 试剂 IV,尽管产率较低。








































 京公网安备 11010802027423号
京公网安备 11010802027423号