当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretically investigating the ability of phenanthroline derivatives to separate transuranic elements and their bonding properties
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2022-07-04 , DOI: 10.1039/d2nj02160a Yiying Zhang 1 , Shouqiang Wu 1 , Anyong Li 1
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2022-07-04 , DOI: 10.1039/d2nj02160a Yiying Zhang 1 , Shouqiang Wu 1 , Anyong Li 1
Affiliation
|
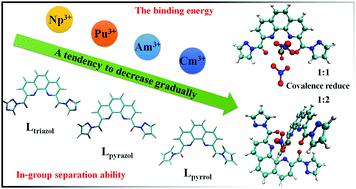
With the development of green chemistry, it is necessary to extract minor actinides from toxic high-level liquid waste. Hence, we need to explore the nature of bonding between ligands and actinide cations in order to design excellent ligands. In the present work, we systematically investigate the potential separation ability of typical tetradentate ligands (1,10-phenanthroline-2,9-diyl)bis((1H-1,2,4-triazol-1-yl)methanone) (Ltriazol), (1,10-phenanthroline-2,9-diyl)bis((1H-pyrazol-1-yl)methanone) (Lpyrazol) and (1,10-phenanthroline-2,9-diyl)bis((1H-pyrrol-1-yl)methanone) (Lpyrrol) for trivalent lanthanides and actinides through quasi-relativistic density functional theory (DFT). The coordination number of the possible extracted complexes of 1 : 1 type AnL(NO3)3 and 1 : 2 type [AnL2(NO3)]2+ (An = Np, Pu, Am, Cm; L = Ltriazol, Lpyrazol, Lpyrrol) is 10 in a nitric acid environment. Both the electrostatic potential and natural atomic charge analyses of the ligands show that Lpyrazol has stronger ability to attract actinide cations than Ltriazol and Lpyrrol, which may be caused by different amide substituents. The QTAIM (quantum theory of atoms in molecules) and IRI (interaction region indicator) analysis validate that there is a weak covalent interaction between the ligand and the actinide metal ion. The analyses including geometry, WBI (Wiberg bond index), NBO (natural bond orbital), and thermodynamics suggest that the interaction between the ligand and actinide cations gradually decreased from Np to Cm. Our theoretical study may contribute to a better understanding of the nature and law of bonding between actinide ions and ligands, and pave the way for designing robust ligands for in-group separation of transuranic complexes in the future.
中文翻译:

从理论上研究菲咯啉衍生物分离超铀元素的能力及其结合特性
随着绿色化学的发展,有必要从有毒的高放废液中提取微量锕系元素。因此,我们需要探索配体与锕系阳离子之间的键合性质,以设计出优良的配体。在目前的工作中,我们系统地研究了典型的四齿配体 (1,10-phenanthroline-2,9-diyl)bis((1 H -1,2,4-triazol-1-yl)methanone) ( L triazol )、(1,10-phenanthroline-2,9-diyl)bis((1 H -pyrazol-1-yl)methanone) (L pyrazol ) 和 (1,10-phenanthroline-2,9-diyl)bis ((1 H-吡咯-1-基)甲酮) (L吡咯) 通过准相对论密度泛函理论 (DFT) 分析三价镧系元素和锕系元素。1 : 1 型 AnL(NO 3 ) 3和 1 : 2 型 [AnL 2 (NO 3 )] 2+ (An = Np, Pu, Am, Cm; L = L triazol , L pyrazol , L pyrrol ) 在硝酸环境中为 10。配体的静电势和自然原子电荷分析表明,L吡唑对锕系阳离子的吸附能力强于L三唑和L吡咯。,这可能是由不同的酰胺取代基引起的。QTAIM(分子中原子的量子理论)和 IRI(相互作用区域指示剂)分析验证了配体和锕系金属离子之间存在弱共价相互作用。几何、WBI(Wiberg 键指数)、NBO(自然键轨道)和热力学分析表明,配体与锕系阳离子之间的相互作用从 Np 逐渐减小到 Cm。我们的理论研究可能有助于更好地理解锕系离子与配体之间的键合性质和规律,并为未来设计用于超铀配合物组内分离的稳健配体铺平道路。
更新日期:2022-07-04
中文翻译:

从理论上研究菲咯啉衍生物分离超铀元素的能力及其结合特性
随着绿色化学的发展,有必要从有毒的高放废液中提取微量锕系元素。因此,我们需要探索配体与锕系阳离子之间的键合性质,以设计出优良的配体。在目前的工作中,我们系统地研究了典型的四齿配体 (1,10-phenanthroline-2,9-diyl)bis((1 H -1,2,4-triazol-1-yl)methanone) ( L triazol )、(1,10-phenanthroline-2,9-diyl)bis((1 H -pyrazol-1-yl)methanone) (L pyrazol ) 和 (1,10-phenanthroline-2,9-diyl)bis ((1 H-吡咯-1-基)甲酮) (L吡咯) 通过准相对论密度泛函理论 (DFT) 分析三价镧系元素和锕系元素。1 : 1 型 AnL(NO 3 ) 3和 1 : 2 型 [AnL 2 (NO 3 )] 2+ (An = Np, Pu, Am, Cm; L = L triazol , L pyrazol , L pyrrol ) 在硝酸环境中为 10。配体的静电势和自然原子电荷分析表明,L吡唑对锕系阳离子的吸附能力强于L三唑和L吡咯。,这可能是由不同的酰胺取代基引起的。QTAIM(分子中原子的量子理论)和 IRI(相互作用区域指示剂)分析验证了配体和锕系金属离子之间存在弱共价相互作用。几何、WBI(Wiberg 键指数)、NBO(自然键轨道)和热力学分析表明,配体与锕系阳离子之间的相互作用从 Np 逐渐减小到 Cm。我们的理论研究可能有助于更好地理解锕系离子与配体之间的键合性质和规律,并为未来设计用于超铀配合物组内分离的稳健配体铺平道路。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号