Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A new chiral stationary phase based on noscapine: Synthesis, enantioseparation, and docking study
Chirality ( IF 2.8 ) Pub Date : 2022-07-01 , DOI: 10.1002/chir.23488 Zohreh Mousavimanesh 1 , Mostafa Shahnani 1 , Amirmohammad Faraji-Shovey 1 , Morteza Bararjanian 1 , Ahmad Shahir Sadr 2, 3 , Alireza Ghassempour 1 , Peyman Salehi 1
Chirality ( IF 2.8 ) Pub Date : 2022-07-01 , DOI: 10.1002/chir.23488 Zohreh Mousavimanesh 1 , Mostafa Shahnani 1 , Amirmohammad Faraji-Shovey 1 , Morteza Bararjanian 1 , Ahmad Shahir Sadr 2, 3 , Alireza Ghassempour 1 , Peyman Salehi 1
Affiliation
|
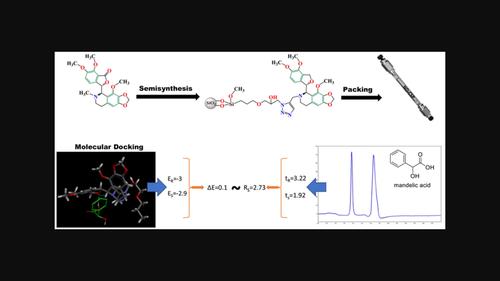
Noscapine is an isolated compound from the opium poppy, with distinctive chiral structure and chemistry, interacts with other compounds due to having multiple π-acceptors, hydrogen bond acceptors, and ionic sites. Therefore, it has promising applicability for the enantioselective separation of a wide range of polar, acidic, basic, and neutral compounds. A new noscapine derivative chiral stationary phase (ND-CSP) has been synthesized by consecutive N-demethylation, reduction, and N-propargylation of noscapine followed by attachment of a solid epoxy-functionalized silica bed through the 1,3-dipolar Huisgen cycloaddition. The noscapine derivative-based stationary phase provides a considerable surface coverage, which is greater than some commercial CSPs and can validate better enantioresolution performance. The major advantages inherent to this chiral selector are stability, reproducibility after more than 200 tests, and substantial loading capacity. The characterization by Fourier transform infrared (FTIR) spectroscopy and elemental analysis indicated successful functionalization of the silica surface. Chromatographic method conditions like flow rate and mobile phase composition for enantioseparation of various compounds such as warfarin, propranolol, mandelic acid, and a sulfanilamide derivative were optimized. Comparing the experimental results with docking data revealed a clear correlation between the calculated binding energy of ND-CSP and each enantiomer with the resolution of enantiomer peaks.
中文翻译:

基于诺卡品的新型手性固定相:合成、对映体分离和对接研究
诺斯卡品是一种从罂粟中分离出来的化合物,具有独特的手性结构和化学性质,由于具有多个 π 受体、氢键受体和离子位点,可与其他化合物相互作用。因此,它在广泛的极性、酸性、碱性和中性化合物的对映选择性分离中具有广阔的应用前景。通过连续的N-去甲基化、还原和N-noscapine 的炔丙基化,然后通过 1,3-偶极 Huisgen 环加成连接固体环氧官能化二氧化硅床。基于 noscine 衍生物的固定相提供了相当大的表面覆盖率,比一些商业 CSP 更大,并且可以验证更好的对映体分离性能。这种手性选择器固有的主要优点是稳定性、200 多次测试后的可重复性和强大的负载能力。傅里叶变换红外 (FTIR) 光谱和元素分析的表征表明二氧化硅表面的成功功能化。对华法林、普萘洛尔、扁桃酸和磺胺衍生物等多种化合物的对映体分离的流速和流动相组成等色谱方法条件进行了优化。
更新日期:2022-07-01
中文翻译:

基于诺卡品的新型手性固定相:合成、对映体分离和对接研究
诺斯卡品是一种从罂粟中分离出来的化合物,具有独特的手性结构和化学性质,由于具有多个 π 受体、氢键受体和离子位点,可与其他化合物相互作用。因此,它在广泛的极性、酸性、碱性和中性化合物的对映选择性分离中具有广阔的应用前景。通过连续的N-去甲基化、还原和N-noscapine 的炔丙基化,然后通过 1,3-偶极 Huisgen 环加成连接固体环氧官能化二氧化硅床。基于 noscine 衍生物的固定相提供了相当大的表面覆盖率,比一些商业 CSP 更大,并且可以验证更好的对映体分离性能。这种手性选择器固有的主要优点是稳定性、200 多次测试后的可重复性和强大的负载能力。傅里叶变换红外 (FTIR) 光谱和元素分析的表征表明二氧化硅表面的成功功能化。对华法林、普萘洛尔、扁桃酸和磺胺衍生物等多种化合物的对映体分离的流速和流动相组成等色谱方法条件进行了优化。






























 京公网安备 11010802027423号
京公网安备 11010802027423号