European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2022-06-30 , DOI: 10.1016/j.ejmech.2022.114575 Huajian Zhu 1 , Lixue Lu 2 , Wenjian Zhu 1 , Yuchen Tan 1 , Yiping Duan 2 , Jie Liu 3 , Wencai Ye 4 , Zheying Zhu 5 , Jinyi Xu 1 , Shengtao Xu 1
|
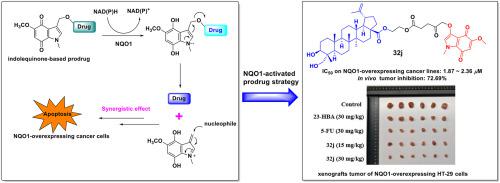
A series of NQO1 selectively activated prodrugs were designed and synthesized by introducing indolequinone moiety to the C-3, C-23 or C-28 position of 23-hydroxybetulinic acid (23-HBA) and its analogues. Among them, the representative compound 32j exhibited significant antiproliferative activities against NQO1-overexpressing HT-29 cells and A549 cells, with IC50 values of 1.87 and 2.36 μM, respectively, which were 20–30-fold more potent than those of parent compound 23-HBA. More importantly, it was demonstrated in the in vivo antitumor experiment that 32j effectively suppressed the tumor volume and largely reduced tumor weight by 72.69% with no apparent toxicity, which was more potent than the positive control 5-fluorouracil. This is the first breakthrough in the improvement of in vivo antitumor activities of 23-HBA derivatives. The further molecular mechanism study revealed that 32j blocked cell cycle arrest at G2/M phase, induced cell apoptosis, depolarized mitochondria and elevated the intracellular ROS levels in a dose-dependent manner. Western blot analysis indicated that 32j induced cell apoptosis by interfering with the expression of apoptosis-related proteins. These findings suggest that compound 32j could be considered as a potent antitumor prodrug candidate which deserves to be further investigated for personalized cancer therapy.
中文翻译:

NAD(P)H的设计与合成:醌氧化还原酶(NQO1)激活的23-羟基桦木酸前药具有增强的抗肿瘤特性
通过将吲哚醌部分引入23-羟基桦木酸(23-HBA)及其类似物的C-3、C-23或C-28位,设计合成了一系列NQO1选择性活化前药。其中,代表化合物32j对 NQO1 过表达的 HT-29 细胞和 A549 细胞具有显着的抗增殖活性,IC 50值分别为 1.87 和 2.36 μM,是母体化合物 23 的 20-30 倍-HBA。更重要的是,体内抗肿瘤实验证明32j有效抑制肿瘤体积,肿瘤重量大幅减少72.69%,无明显毒性,比阳性对照5-氟尿嘧啶更有效。这是提高23-HBA衍生物体内抗肿瘤活性的第一个突破。进一步的分子机制研究表明,32j在 G2/M 期阻断细胞周期停滞、诱导细胞凋亡、去极化线粒体并以剂量依赖性方式提高细胞内 ROS 水平。Western印迹分析表明,32j通过干扰细胞凋亡相关蛋白的表达诱导细胞凋亡。这些发现表明化合物32j可以被认为是一种有效的抗肿瘤前药候选物,值得进一步研究用于个体化癌症治疗。






























 京公网安备 11010802027423号
京公网安备 11010802027423号