Nano Research ( IF 9.5 ) Pub Date : 2022-06-29 , DOI: 10.1007/s12274-022-4598-6 Madiha Zahra Syeda , Tu Hong , Min Zhang , Yanfei Han , Xiaoling Zhu , Songmin Ying , Longguang Tang
|
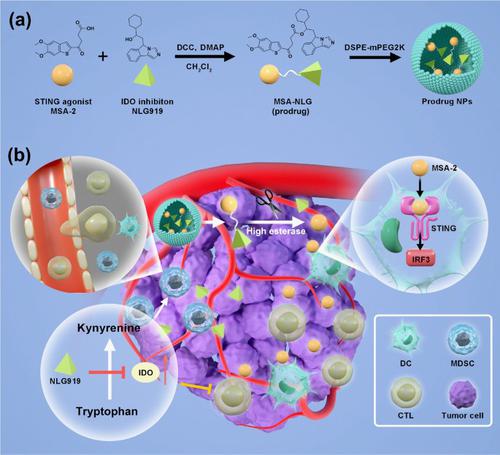
Cancer immunotherapy has made significant progress in the last few decades, revolutionizing oncology. However, low patient response rates and potential immune-related adverse events continue to be major clinical challenges. Cancer nanomedicine, by virtue of its regulated delivery and modular flexibility, has shown the potential to strengthen antitumor immune responses and sensitize tumors to immunotherapy. In this study, we developed tumor microenvironment (TME) responsive nanomedicine to achieve specific and localized amplification of the immune response in tumor tissue in a safe and effective manner, while simultaneously reducing immune-related side effects. We synthesized the TME responsive prodrug by coupling MSA-2, a stimulator of interferon genes (STING) agonist, and NLG-919, an indoleamine 2,3 dioxygenase (IDO) inhibitor. The prodrug was assembled into nanoparticles to enhance the solubility and bioavailability. By synthesizing a TME responsive prodrug, we aim to explore the therapeutic efficacy of combined regimen (STING agonist and IDO inhibitor) for cancer, and reduce the unwanted side effects of STING agonism on normal tissues. Free prodrug and nanoparticles were characterized by mass spectrometry, dynamic light scattering (DLS), and transmission electron microscopy (TEM). Following that, we investigated the tumor accumulation, anti-tumor activity, and toxicity in vitro and in vivo. Prodrug nanoparticles demonstrated the ability to inhibit the tumor growth and activate antitumor immune response by modulating immune cells populations in tumor microenvironment. The TME responsive nanomedicine provided an effective tool for precise targeting, promoting antitumor immunity, and efficient tumor growth inhibition with safety. Outcomes of this study may have implications for future clinical trials.
中文翻译:

通过 STING 激动剂和 IDO 抑制剂酯化的前药纳米平台用于协同癌症免疫治疗
在过去的几十年中,癌症免疫疗法取得了重大进展,彻底改变了肿瘤学。然而,低患者反应率和潜在的免疫相关不良事件仍然是主要的临床挑战。癌症纳米药物凭借其可调节的递送和模块化的灵活性,已显示出增强抗肿瘤免疫反应并使肿瘤对免疫疗法敏感的潜力。在这项研究中,我们开发了肿瘤微环境(TME)响应性纳米药物,以安全有效的方式实现肿瘤组织中免疫反应的特异性和局部放大,同时减少免疫相关的副作用。我们通过偶联干扰素基因 (STING) 激动剂 MSA-2 和吲哚胺 2,3 双加氧酶 (IDO) 抑制剂 NLG-919 合成了 TME 响应性前药。将前药组装成纳米颗粒以提高溶解度和生物利用度。通过合成 TME 反应性前药,我们旨在探索联合方案(STING 激动剂和 IDO 抑制剂)对癌症的治疗效果,并减少 STING 激动剂对正常组织的不良副作用。游离前药和纳米颗粒通过质谱、动态光散射 (DLS) 和透射电子显微镜 (TEM) 进行表征。之后,我们研究了肿瘤积累、抗肿瘤活性和毒性 游离前药和纳米颗粒通过质谱、动态光散射 (DLS) 和透射电子显微镜 (TEM) 进行表征。之后,我们研究了肿瘤积累、抗肿瘤活性和毒性 游离前药和纳米颗粒通过质谱、动态光散射 (DLS) 和透射电子显微镜 (TEM) 进行表征。之后,我们研究了肿瘤积累、抗肿瘤活性和毒性体外和体内。前药纳米颗粒通过调节肿瘤微环境中的免疫细胞群,显示出抑制肿瘤生长和激活抗肿瘤免疫反应的能力。TME响应性纳米药物为精准靶向、促进抗肿瘤免疫和安全有效地抑制肿瘤生长提供了有效工具。这项研究的结果可能对未来的临床试验产生影响。













































 京公网安备 11010802027423号
京公网安备 11010802027423号