当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phenylsulfamoyl Benzoic Acid Inhibitor of ERAP2 with a Novel Mode of Inhibition
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2022-06-29 , DOI: 10.1021/acschembio.2c00093 Richa Arya 1 , Zachary Maben 1 , Digamber Rane 2 , Akbar Ali 3 , Lawrence J Stern 1, 3
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2022-06-29 , DOI: 10.1021/acschembio.2c00093 Richa Arya 1 , Zachary Maben 1 , Digamber Rane 2 , Akbar Ali 3 , Lawrence J Stern 1, 3
Affiliation
|
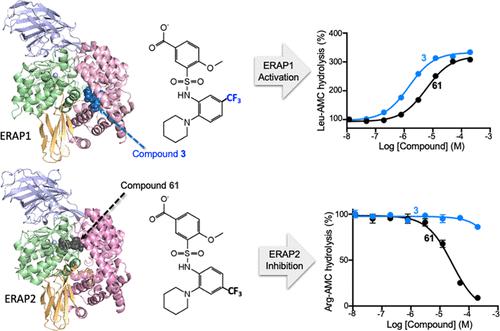
ERAP1 and ERAP2 are endoplasmic reticulum zinc-binding aminopeptidases that play crucial roles in processing peptides for loading onto class I major histocompatibility complex proteins. These enzymes are therapeutic targets in cancer and autoimmune disorders. The discovery of inhibitors specific to ERAP1 or ERAP2 has been challenging due to the similarity in their active site residues and domain architectures. Here, we identify 4-methoxy-3-{[2-piperidin-1-yl-4-(trifluoromethyl) phenyl] sulfamoyl} benzoic acid (compound 61) as a novel inhibitor of ERAP2 and determine the crystal structure of ERAP2 bound to compound 61. Compound 61 binds near the catalytic center of ERAP2, at a distinct site from previously known peptidomimetic inhibitors, and inhibits by an uncompetitive mechanism. Surprisingly, for ERAP1, compound 61 was found to activate model substrate hydrolysis, similarly to the previously characterized 5-trifluoromethyl regioisomer of compound 61, known as compound 3. We characterized the specificity determinants of ERAP1 and ERAP2 that control the binding of compounds 3 and 61. At the active site of ERAP1, Lys380 in the S1′ pocket is a key determinant for the binding of both compounds 3 and 61. At the allosteric site, ERAP1 binds either compound, leading to the activation of model substrate hydrolysis. Although ERAP2 substrate hydrolysis is not activated by either compound, the mutation of His904 to alanine reveals a cryptic allosteric site that allows for the activation by compound 3. Thus, we have identified selectivity determinants in the active and allosteric sites of ERAP2 that govern the binding of two similar compounds, which potentially could be exploited to develop more potent and specific inhibitors.
中文翻译:

具有新型抑制模式的 ERAP2 苯氨磺酰苯甲酸抑制剂
ERAP1 和 ERAP2 是内质网锌结合氨肽酶,在处理肽以加载到 I 类主要组织相容性复合物蛋白中起关键作用。这些酶是癌症和自身免疫性疾病的治疗靶点。由于其活性位点残基和结构域结构的相似性,ERAP1 或 ERAP2 特异性抑制剂的发现一直具有挑战性。在这里,我们确定了 4-methoxy-3-{[2-piperidin-1-yl-4-(trifluoromethyl) phenyl] sulfamoyl} benzoic acid (compound 61 ) 作为 ERAP2 的新型抑制剂,并确定了 ERAP2 的晶体结构与化合物61。化合物61在 ERAP2 的催化中心附近结合,在与先前已知的拟肽抑制剂不同的位点,并通过非竞争机制进行抑制。令人惊讶的是,对于 ERAP1,发现化合物61激活模型底物水解,类似于先前表征的化合物61的 5-三氟甲基区域异构体,称为化合物3。我们表征了 ERAP1 和 ERAP2 的特异性决定因素,它们控制化合物3和61 . 在 ERAP1 的活性位点,S1' 口袋中的 Lys380 是化合物3和61结合的关键决定因素. 在变构位点,ERAP1 结合任一化合物,导致模型底物水解的激活。尽管 ERAP2 底物水解不会被任何一种化合物激活,但 His904 向丙氨酸的突变揭示了一个隐秘的变构位点,允许化合物3激活。因此,我们已经确定了 ERAP2 的活性和变构位点中控制两种相似化合物结合的选择性决定因素,这可能被用于开发更有效和特异性的抑制剂。
更新日期:2022-06-29
中文翻译:

具有新型抑制模式的 ERAP2 苯氨磺酰苯甲酸抑制剂
ERAP1 和 ERAP2 是内质网锌结合氨肽酶,在处理肽以加载到 I 类主要组织相容性复合物蛋白中起关键作用。这些酶是癌症和自身免疫性疾病的治疗靶点。由于其活性位点残基和结构域结构的相似性,ERAP1 或 ERAP2 特异性抑制剂的发现一直具有挑战性。在这里,我们确定了 4-methoxy-3-{[2-piperidin-1-yl-4-(trifluoromethyl) phenyl] sulfamoyl} benzoic acid (compound 61 ) 作为 ERAP2 的新型抑制剂,并确定了 ERAP2 的晶体结构与化合物61。化合物61在 ERAP2 的催化中心附近结合,在与先前已知的拟肽抑制剂不同的位点,并通过非竞争机制进行抑制。令人惊讶的是,对于 ERAP1,发现化合物61激活模型底物水解,类似于先前表征的化合物61的 5-三氟甲基区域异构体,称为化合物3。我们表征了 ERAP1 和 ERAP2 的特异性决定因素,它们控制化合物3和61 . 在 ERAP1 的活性位点,S1' 口袋中的 Lys380 是化合物3和61结合的关键决定因素. 在变构位点,ERAP1 结合任一化合物,导致模型底物水解的激活。尽管 ERAP2 底物水解不会被任何一种化合物激活,但 His904 向丙氨酸的突变揭示了一个隐秘的变构位点,允许化合物3激活。因此,我们已经确定了 ERAP2 的活性和变构位点中控制两种相似化合物结合的选择性决定因素,这可能被用于开发更有效和特异性的抑制剂。






























 京公网安备 11010802027423号
京公网安备 11010802027423号