当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
N-Substituted Arylidene-3-(Methylsulfonyl)-2-Oxoimidazolidine-1-Carbohydrazide as Cholinesterase Inhibitors: Design, Synthesis, and Molecular Docking Study
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2022-06-27 , DOI: 10.1002/cbdv.202200265 Fatih Tok 1 , Begüm Nurpelin Sağlık 2, 3 , Yusuf Özkay 2, 3 , Zafer Asım Kaplancıklı 2 , Bedia Koçyiğit-Kaymakçıoğlu 1
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2022-06-27 , DOI: 10.1002/cbdv.202200265 Fatih Tok 1 , Begüm Nurpelin Sağlık 2, 3 , Yusuf Özkay 2, 3 , Zafer Asım Kaplancıklı 2 , Bedia Koçyiğit-Kaymakçıoğlu 1
Affiliation
|
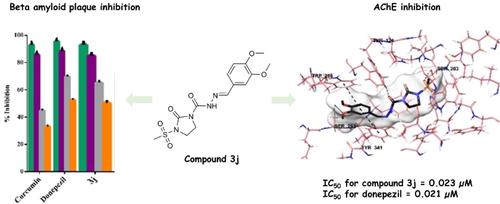
The development of new enzyme inhibitors in degenerative brain diseases has gained more attention. Enzyme inhibitors play an effective role in controlling central nervous system diseases. For this purpose, a novel series of hydrazone derivatives containing imidazolidine ring aimed against Alzheimer's disease (AD), have been designed and synthesized. The acetylcholinesterase (AChE) enzyme inhibitory activity of these compounds was investigated. The structures of the compounds were determined by IR, 1H and 13C-NMR and mass spectroscopic methods. Inhibition studies on the cholinesterase (ChE) enzymes and β-amyloid plaque inhibition test of the compounds were performed. Based on the experimental results, compound 3j bearing dimethoxy substituent on the aromatic ring like donepezil exhibited the most AChE inhibitory activity with the IC50 values of 0.023±0.001 μM. Owing to obtained biological activity and molecular docking study results, it is thought that the most active compound 3j may play a role in both symptomatic and palliative treatment of AD.
中文翻译:

N-取代亚芳基-3-(甲基磺酰基)-2-氧代咪唑烷-1-碳酰肼作为胆碱酯酶抑制剂:设计、合成和分子对接研究
在退行性脑病中开发新的酶抑制剂得到了更多的关注。酶抑制剂在控制中枢神经系统疾病方面发挥着有效作用。为此,已经设计和合成了一系列新的含有咪唑烷环的腙衍生物,旨在对抗阿尔茨海默病(AD)。研究了这些化合物的乙酰胆碱酯酶 (AChE) 酶抑制活性。化合物的结构通过IR、1 H和13 C-NMR和质谱法确定。对化合物的胆碱酯酶(ChE)酶和β-淀粉样蛋白斑块抑制试验进行了抑制研究。根据实验结果,化合物3j在芳环上带有二甲氧基取代基的多奈哌齐表现出最强的AChE抑制活性,IC 50值为0.023±0.001 μM。由于获得的生物活性和分子对接研究结果,认为活性最强的化合物3j可能在AD的对症和姑息治疗中发挥作用。
更新日期:2022-06-27
中文翻译:

N-取代亚芳基-3-(甲基磺酰基)-2-氧代咪唑烷-1-碳酰肼作为胆碱酯酶抑制剂:设计、合成和分子对接研究
在退行性脑病中开发新的酶抑制剂得到了更多的关注。酶抑制剂在控制中枢神经系统疾病方面发挥着有效作用。为此,已经设计和合成了一系列新的含有咪唑烷环的腙衍生物,旨在对抗阿尔茨海默病(AD)。研究了这些化合物的乙酰胆碱酯酶 (AChE) 酶抑制活性。化合物的结构通过IR、1 H和13 C-NMR和质谱法确定。对化合物的胆碱酯酶(ChE)酶和β-淀粉样蛋白斑块抑制试验进行了抑制研究。根据实验结果,化合物3j在芳环上带有二甲氧基取代基的多奈哌齐表现出最强的AChE抑制活性,IC 50值为0.023±0.001 μM。由于获得的生物活性和分子对接研究结果,认为活性最强的化合物3j可能在AD的对症和姑息治疗中发挥作用。































 京公网安备 11010802027423号
京公网安备 11010802027423号