当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
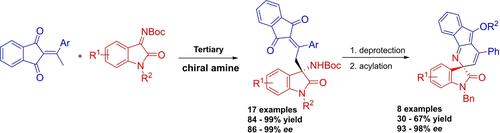
Asymmetric Sequential Vinylogous Mannich/Annulation/Acylation Process of 2-Ethylidene 1,3-Indandiones and Isatin N-Boc Ketimines: Access to Chiral Spiro-Oxindole Piperidine Derivatives
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-06-27 , DOI: 10.1002/adsc.202200465 I-Ting Chen, Ren-You Guan, Jeng-Liang Han
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-06-27 , DOI: 10.1002/adsc.202200465 I-Ting Chen, Ren-You Guan, Jeng-Liang Han
|
A catalytic asymmetric vinylogous Mannich/annulation/acylation reaction of 2-ethylidene 1,3-indandiones with isatin N-Boc ketimines has been developed, which furnished a series of chiral spiro-oxindole piperidine derivatives with polyaromatic scaffolds in 30–67% yield and 93–98% ee. The DFT computational calculation is used to probe and explain the origin of the enantioselectivity and observed formation of O-acetylated products.
中文翻译:

2-亚乙基 1,3-茚满二酮和靛红 N-Boc 酮亚胺的不对称序列乙烯基曼尼希/环化/酰化过程:获得手性螺氧吲哚哌啶衍生物
开发了 2-亚乙基 1,3-茚满二酮与靛红N -Boc 酮亚胺的催化不对称插烯曼尼希/环化/酰化反应,以 30-67% 的产率和93–98% EE。DFT计算计算用于探测和解释对映选择性的起源和观察到的O-乙酰化产物的形成。
更新日期:2022-06-27
中文翻译:

2-亚乙基 1,3-茚满二酮和靛红 N-Boc 酮亚胺的不对称序列乙烯基曼尼希/环化/酰化过程:获得手性螺氧吲哚哌啶衍生物
开发了 2-亚乙基 1,3-茚满二酮与靛红N -Boc 酮亚胺的催化不对称插烯曼尼希/环化/酰化反应,以 30-67% 的产率和93–98% EE。DFT计算计算用于探测和解释对映选择性的起源和观察到的O-乙酰化产物的形成。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号