当前位置:
X-MOL 学术
›
Acta Cryst. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, X-ray structure, antimicrobial activity, DFT and molecular docking studies of N-(thiophen-2-ylmethyl)thiophene-2-carboxamide
Acta Crystallographica Section C ( IF 0.7 ) Pub Date : 2022-06-23 , DOI: 10.1107/s2053229622006283 Şükriye Çakmak 1 , Zeynep Demircioğlu 2 , Serap Uzun 3 , Aysel Veyisoğlu 1 , Hasan Yakan 4 , Cem Cüneyt Ersanli 2
Acta Crystallographica Section C ( IF 0.7 ) Pub Date : 2022-06-23 , DOI: 10.1107/s2053229622006283 Şükriye Çakmak 1 , Zeynep Demircioğlu 2 , Serap Uzun 3 , Aysel Veyisoğlu 1 , Hasan Yakan 4 , Cem Cüneyt Ersanli 2
Affiliation
|
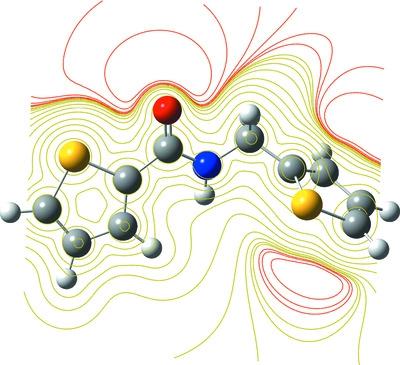
In the present study, N-(thiophen-2-ylmethyl)thiophene-2-carboxamide, C10H9NOS2, (I), was obtained by the reaction of thiophene-2-carbonyl chloride and thiophen-2-ylmethanamine. Characterization of (I) was carried out using X-ray diffraction, spectroscopic techniques and elemental analyses. The DFT/B3LYP/6-311++G(d,p) theoretical level was successfully applied to calculate the optimized geometry and the local and global chemical activity parameters. The results obtained show good agreement between the experimental and theoretical geometrical parameters. The local and global chemical activity parameters were examined to determine the electrophilic and nucleophilic sites in (I). The natural bond orbital (NBO) analysis of (I) gives an efficient methodology for investigating the inter- and intramolecular bonding, as well as giving a convenient basis for investigating charge transfer or conjugative interactions in molecular systems. Also, the antimicrobial activity of (I) was investigated against eight microorganisms using the microdilution method and it is found to have an effective antibacterial activity. In addition, molecular docking studies were calculated in order to understand the nature of the binding of (I) with a lung cancer protein (PDB entry 1x2j).
中文翻译:

N-(噻吩-2-基甲基)噻吩-2-甲酰胺的合成、X射线结构、抗菌活性、DFT和分子对接研究
在本研究中,N- (噻吩-2-基甲基)噻吩-2-甲酰胺,C 10 H 9 NOS 2, (I), 由噻吩-2-羰基氯和噻吩-2-基甲胺反应制得。(I) 的表征使用 X 射线衍射、光谱技术和元素分析进行。DFT/B3LYP/6-311++G(d,p)理论水平成功地应用于计算优化几何和局部和全局化学活性参数。获得的结果表明实验和理论几何参数之间的一致性很好。检查局部和全局化学活性参数以确定(I)中的亲电和亲核位点。(I) 的自然键轨道 (NBO) 分析提供了一种研究分子间和分子内键合的有效方法,以及为研究分子系统中的电荷转移或共轭相互作用提供方便的基础。此外,使用微量稀释法研究了(I)对8种微生物的抗菌活性,发现它具有有效的抗菌活性。此外,计算分子对接研究以了解 (I) 与肺癌蛋白 (PDB 条目 1x2j) 结合的性质。
更新日期:2022-06-23
中文翻译:

N-(噻吩-2-基甲基)噻吩-2-甲酰胺的合成、X射线结构、抗菌活性、DFT和分子对接研究
在本研究中,N- (噻吩-2-基甲基)噻吩-2-甲酰胺,C 10 H 9 NOS 2, (I), 由噻吩-2-羰基氯和噻吩-2-基甲胺反应制得。(I) 的表征使用 X 射线衍射、光谱技术和元素分析进行。DFT/B3LYP/6-311++G(d,p)理论水平成功地应用于计算优化几何和局部和全局化学活性参数。获得的结果表明实验和理论几何参数之间的一致性很好。检查局部和全局化学活性参数以确定(I)中的亲电和亲核位点。(I) 的自然键轨道 (NBO) 分析提供了一种研究分子间和分子内键合的有效方法,以及为研究分子系统中的电荷转移或共轭相互作用提供方便的基础。此外,使用微量稀释法研究了(I)对8种微生物的抗菌活性,发现它具有有效的抗菌活性。此外,计算分子对接研究以了解 (I) 与肺癌蛋白 (PDB 条目 1x2j) 结合的性质。

































 京公网安备 11010802027423号
京公网安备 11010802027423号