当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Djurleite Copper Sulfide-Coupled Cobalt Sulfide Interface for a Stable and Efficient Electrocatalyst
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2022-06-28 , DOI: 10.1021/acsami.2c06010 Nandapriya Manivelan 1 , Senthil Karuppanan 2 , Kandasamy Prabakar 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2022-06-28 , DOI: 10.1021/acsami.2c06010 Nandapriya Manivelan 1 , Senthil Karuppanan 2 , Kandasamy Prabakar 1
Affiliation
|
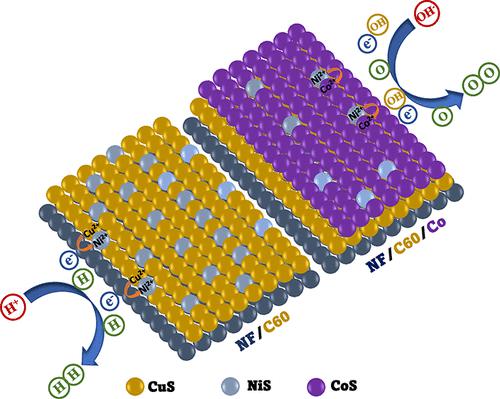
Transition metal sulfides (TMS) exhibit proliferated edge sites, facile electrode kinetics, and improved intrinsic electrical conductivity, which demand low potential requirements for total water splitting application. Here, we have propounded copper sulfide-coupled cobalt sulfide nanosheets grown on 3D nickel as an electrocatalyst for hydrogen (HER) and oxygen evolution (OER) reactions. The formation of djurleite copper sulfide with a Cu vacancy enables faster H+ ion transport and shows improved HER activity with a remarkably lower overpotential of 164 mV at 10 mA/cm2, whereas cobalt-incorporated copper sulfide undergoes cation exchange during synthesis and shows elevated OER activity with a lower overpotential of 240 mV at 10 mA/cm2 for the OER. Moreover, Cu2–xS/Co is said to have a hybrid CoS–CoS2 interface and provide Co2+ active sites on the surface and enable the fast adsorption of intermediate species (OH*, O*, and OOH*), which lowers the potential requirement. The copper vacancy and cation exchange with a hybrid CoS–CoS2 structure are helpful in supplying more surface reactive species and faster ion transport for the HER and OER, respectively. The full-cell electrolyzer requires a very low potential of 1.58 V to attain a current density of 10 mA/cm2, and it shows excellent stability for 50 h at 100 mA/cm2 as confirmed by the chronopotentiometry test.
中文翻译:

Djurleite 硫化铜偶联硫化钴界面用于稳定高效的电催化剂
过渡金属硫化物 (TMS) 具有增殖的边缘位点、容易的电极动力学和改进的本征电导率,这对全水分解应用的电位要求较低。在这里,我们提出了在 3D 镍上生长的硫化铜偶联硫化钴纳米片作为氢 (HER) 和析氧 (OER) 反应的电催化剂。形成具有 Cu 空位的 djurleite 硫化铜能够更快地传输 H +离子,并显示出改善的 HER 活性,在 10 mA/cm 2下过电位显着降低 164 mV ,而掺入钴的硫化铜在合成过程中经历阳离子交换并显示出升高的OER 活性在 10 mA/cm 2时具有 240 mV 的较低过电位对于开放式教育资源。此外,据说 Cu 2– x S/Co 具有混合 CoS–CoS 2界面并在表面提供 Co 2+活性位点,并能够快速吸附中间物质(OH*、O* 和 OOH*),这降低了潜在的要求。具有混合 CoS-CoS 2结构的铜空位和阳离子交换有助于分别为 HER 和 OER 提供更多的表面活性物质和更快的离子传输。全电池电解槽需要非常低的 1.58 V 电位才能达到 10 mA/cm 2的电流密度,并且通过计时电位测试证实,它在 100 mA/cm 2下表现出出色的 50 小时稳定性。
更新日期:2022-06-28
中文翻译:

Djurleite 硫化铜偶联硫化钴界面用于稳定高效的电催化剂
过渡金属硫化物 (TMS) 具有增殖的边缘位点、容易的电极动力学和改进的本征电导率,这对全水分解应用的电位要求较低。在这里,我们提出了在 3D 镍上生长的硫化铜偶联硫化钴纳米片作为氢 (HER) 和析氧 (OER) 反应的电催化剂。形成具有 Cu 空位的 djurleite 硫化铜能够更快地传输 H +离子,并显示出改善的 HER 活性,在 10 mA/cm 2下过电位显着降低 164 mV ,而掺入钴的硫化铜在合成过程中经历阳离子交换并显示出升高的OER 活性在 10 mA/cm 2时具有 240 mV 的较低过电位对于开放式教育资源。此外,据说 Cu 2– x S/Co 具有混合 CoS–CoS 2界面并在表面提供 Co 2+活性位点,并能够快速吸附中间物质(OH*、O* 和 OOH*),这降低了潜在的要求。具有混合 CoS-CoS 2结构的铜空位和阳离子交换有助于分别为 HER 和 OER 提供更多的表面活性物质和更快的离子传输。全电池电解槽需要非常低的 1.58 V 电位才能达到 10 mA/cm 2的电流密度,并且通过计时电位测试证实,它在 100 mA/cm 2下表现出出色的 50 小时稳定性。































 京公网安备 11010802027423号
京公网安备 11010802027423号