当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spectral−Structural Effects of the Keto−Enol−Enolate and Phenol−Phenolate Equilibria of Oxyluciferin
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2010-08-25 , DOI: 10.1021/ja102885g Panče Naumov 1 , Manoj Kochunnoonny 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2010-08-25 , DOI: 10.1021/ja102885g Panče Naumov 1 , Manoj Kochunnoonny 1
Affiliation
|
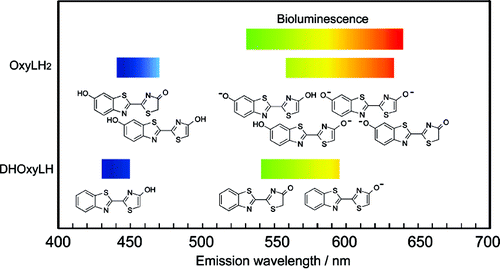
The effects of environmental polarity on the enolization of the keto form and the deprotonation of the enol, and the role of the neutral and ionized 6'-OH group in the fluorescence of the firefly emitter, oxyluciferin, were assessed through a detailed study of the structure and absorption and fluorescence spectra of its 6'-dehydroxylated analogue. It was found that the deprotonated 6'-O(-) group is a necessary, albeit insufficient, factor in accounting for the observed yellow-green and red emissions of oxyluciferin. Its negative charge is essential for effective excited-state charge transfer, which lowers the emission energy and broadens the emission spectrum. Deprotonation of the 6'-OH group changes its effect on the emission energy from blue- to red-shifting. Furthermore, the combination of these opposite effects and resonance stabilization of the phenolate-keto form causes switching of the order of maximum emission wavelengths of the three species involved in the keto-enol-enolate equilibrium from enol << keto < enolate in absence of 6'-OH to keto < enol << enolate with 6'-OH, to enol < enolate < keto with 6'-O(-). If only the keto-enol-enolate equilibrium is considered, solvents of medium polarity are the most effective in decreasing the excited-state energy. Polar or very polar environments also stimulate shift of the ground-state equilibrium toward the enol form. Under such circumstances, the enol group can be partly or completely deprotonated in the ground state or from the excited state: a polar environment facilitates the ionization, while a less polar environment requires the presence of a stronger base. In the absence of bases, the ground-state keto form exists only in solvents of very weak to medium polarity, but with stronger bases, it can also exist in a nonpolar or very weakly polar environment, usually together with the enolate anion. The phenol-enolate form of oxyluciferin, a species that could not be experimentally detected prior to this study, was identified as a yellow-emitting species.
中文翻译:

氧化荧光素的酮-烯醇-烯醇盐和苯酚-酚盐平衡的光谱-结构效应
环境极性对酮形式的烯醇化和烯醇的去质子化的影响,以及中性和电离的 6'-OH 基团在萤火虫发射体氧化荧光素的荧光中的作用,通过对其 6'-脱羟基类似物的结构、吸收和荧光光谱。发现去质子化的 6'-O(-) 组是一个必要的因素,尽管不够充分,可以解释观察到的氧化荧光素的黄绿色和红色排放。它的负电荷对于有效的激发态电荷转移是必不可少的,这降低了发射能量并拓宽了发射光谱。6'-OH 基团的去质子化改变了它对发射能量的影响,从蓝移到红移。此外,这些相反的效应和酚盐-酮形式的共振稳定性的组合导致参与酮-烯醇-烯醇平衡的三种物质的最大发射波长的顺序从烯醇 << 酮 < 烯醇在没有 6'- OH 到酮 < 烯醇 << 与 6'-OH 烯醇化,到烯醇 < 烯醇 < 酮与 6'-O(-)。如果只考虑酮-烯醇-烯醇平衡,中等极性的溶剂在降低激发态能量方面最有效。极性或非常极性的环境也会刺激基态平衡向烯醇形式的转变。在这种情况下,烯醇基团可以在基态或激发态部分或完全去质子化:极性环境有利于电离,而较少极性的环境需要有更强的基础。在没有碱的情况下,基态酮形式仅存在于极性非常弱到中等极性的溶剂中,但如果有较强的碱,它也可以存在于非极性或极性非常弱的环境中,通常与烯醇阴离子一起存在。氧化荧光素的酚-烯醇化物形式是一种在本研究之前无法通过实验检测到的物种,被确定为发黄光物种。
更新日期:2010-08-25
中文翻译:

氧化荧光素的酮-烯醇-烯醇盐和苯酚-酚盐平衡的光谱-结构效应
环境极性对酮形式的烯醇化和烯醇的去质子化的影响,以及中性和电离的 6'-OH 基团在萤火虫发射体氧化荧光素的荧光中的作用,通过对其 6'-脱羟基类似物的结构、吸收和荧光光谱。发现去质子化的 6'-O(-) 组是一个必要的因素,尽管不够充分,可以解释观察到的氧化荧光素的黄绿色和红色排放。它的负电荷对于有效的激发态电荷转移是必不可少的,这降低了发射能量并拓宽了发射光谱。6'-OH 基团的去质子化改变了它对发射能量的影响,从蓝移到红移。此外,这些相反的效应和酚盐-酮形式的共振稳定性的组合导致参与酮-烯醇-烯醇平衡的三种物质的最大发射波长的顺序从烯醇 << 酮 < 烯醇在没有 6'- OH 到酮 < 烯醇 << 与 6'-OH 烯醇化,到烯醇 < 烯醇 < 酮与 6'-O(-)。如果只考虑酮-烯醇-烯醇平衡,中等极性的溶剂在降低激发态能量方面最有效。极性或非常极性的环境也会刺激基态平衡向烯醇形式的转变。在这种情况下,烯醇基团可以在基态或激发态部分或完全去质子化:极性环境有利于电离,而较少极性的环境需要有更强的基础。在没有碱的情况下,基态酮形式仅存在于极性非常弱到中等极性的溶剂中,但如果有较强的碱,它也可以存在于非极性或极性非常弱的环境中,通常与烯醇阴离子一起存在。氧化荧光素的酚-烯醇化物形式是一种在本研究之前无法通过实验检测到的物种,被确定为发黄光物种。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号