当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mild Amide Synthesis Using Nitrobenzene under Neutral Conditions
Organic Letters ( IF 4.9 ) Pub Date : 2022-06-27 , DOI: 10.1021/acs.orglett.2c01743 Ni Xiong 1 , Yuanqi Dong 1 , Bin Xu 1 , Yang Li 1 , Rong Zeng 1, 2
Organic Letters ( IF 4.9 ) Pub Date : 2022-06-27 , DOI: 10.1021/acs.orglett.2c01743 Ni Xiong 1 , Yuanqi Dong 1 , Bin Xu 1 , Yang Li 1 , Rong Zeng 1, 2
Affiliation
|
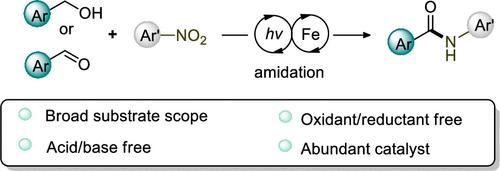
Amide synthesis is one of the most important transformations in organic chemistry due to the broad application in pharmaceutical drugs and organic materials. In this report, we describe a mild protocol for amide formation using the readily available nitroarenes as nitrogen sources and an inexpensive iron complex as a catalyst. Because of the use of the pH-neutral conditions and the avoidance of the strong oxidant or reductant, a wide range of aromatic and aliphatic aldehydes as well as nitroarenes with various functional groups could be tolerated well. A plausible mechanism is proposed based on the detailed studies, in which iron catalyst initiates the radical process and the solvent plays a key role as O-atom acceptor.
中文翻译:

中性条件下硝基苯合成温和酰胺
由于在药物和有机材料中的广泛应用,酰胺合成是有机化学中最重要的转化之一。在本报告中,我们描述了一种温和的酰胺形成方案,使用现成的硝基芳烃作为氮源和廉价的铁络合物作为催化剂。由于使用 pH 中性条件和避免强氧化剂或还原剂,可以很好地耐受各种芳香族和脂肪族醛以及具有各种官能团的硝基芳烃。在详细研究的基础上提出了一种合理的机制,其中铁催化剂引发自由基过程,溶剂作为 O 原子受体起关键作用。
更新日期:2022-06-27
中文翻译:

中性条件下硝基苯合成温和酰胺
由于在药物和有机材料中的广泛应用,酰胺合成是有机化学中最重要的转化之一。在本报告中,我们描述了一种温和的酰胺形成方案,使用现成的硝基芳烃作为氮源和廉价的铁络合物作为催化剂。由于使用 pH 中性条件和避免强氧化剂或还原剂,可以很好地耐受各种芳香族和脂肪族醛以及具有各种官能团的硝基芳烃。在详细研究的基础上提出了一种合理的机制,其中铁催化剂引发自由基过程,溶剂作为 O 原子受体起关键作用。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号