Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2022-06-27 , DOI: 10.1016/j.bioorg.2022.105991 Hua-Tao Liu 1 , Chun-Yue Weng 1 , Shen-Yuan Xu 1 , Shu-Fang Li 1 , Ya-Jun Wang 1 , Yu-Guo Zheng 1
|
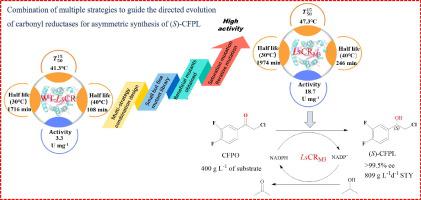
Traditional screening methods of enzyme engineering often require building large mutant libraries to screen for potentially beneficial sites, which are often time-consuming and labor-intensive with low mining efficiency. In this study, a novel enzyme engineering strategy was established to modify carbonyl reductase LsCR for the synthesis of (1S)-2-chloro-1-(3,4-difluorophenyl) ethanol ((S)-CFPL), which is a key intermediate of anticoagulant drug ticagrelor. The strategy was developed by combining HotSpot, FireProt and multiple sequence alignment, resulting in the construction of a “small and smart” mutant library including 10 mutations. Among them, 5 mutations were positive, resulting in a 50% mining accuracy of beneficial sites. Finally, a highly active mutant LsCRM3 (N101D/A117G/F147L) was obtained by further screening through saturation mutation and iterative mutation. Compared with wild type (WT) LsCR, the catalytic activity of LsCRM3 was increased by 4.7 times, the catalytic efficiency kcat/KM value was increased by 2.9 times, and the half-life t1/2 at 40 °C was increased by 1.3 times. Due to the low aqueous solubility of the substrate 2-chloro-1-(3,4-difluorophenyl) ethanone (CFPO), isopropanol was used as not only the co-substrate but also co-solvent. In the presence of 40% (v/v) isopropanol, LsCRM3 completely reduced 400 g/L CFPO to enantiomerically pure CFPL (99.9%, e.e.) in 11 h with a space–time yield (STY) as high as 809 g/L∙d.
中文翻译:

羰基还原酶 LsCR 的定向进化用于 (1S)-2-氯-1-(3,4-二氟苯基) 乙醇的对映选择性合成
酶工程的传统筛选方法通常需要构建大型突变库来筛选潜在的有益位点,这通常耗时耗力且挖掘效率低。在这项研究中,建立了一种新的酶工程策略来修饰羰基还原酶Ls CR 以合成 (1 S )-2-chloro-1-(3,4-difluorophenyl) 乙醇 (( S)-CFPL),是抗凝药物替格瑞洛的关键中间体。该策略是通过结合 HotSpot、FireProt 和多序列比对来开发的,从而构建了一个包含 10 个突变的“小而智能”的突变文库。其中,5个突变为阳性,导致有益位点挖掘准确率达到50%。最后,通过饱和突变和迭代突变进一步筛选得到高活性突变体Ls CR M3 (N101D/A117G/F147L)。与野生型(WT)Ls CR相比, Ls CR M3的催化活性提高了4.7倍,催化效率k cat / K M值提高了2.9倍, 40℃时的半衰期t 1/2提高了1.3倍。由于底物 2-氯-1-(3,4-二氟苯基) 乙酮 (CFPO) 的水溶性低,异丙醇不仅用作共底物,而且还用作共溶剂。在 40% ( v / v ) 异丙醇存在下,Ls CR M3在 11 小时内将 400 g/L CFPO 完全还原为对映体纯 CFPL (99.9%, ee ),时空产率 (STY) 高达 809 g /L∙d。






























 京公网安备 11010802027423号
京公网安备 11010802027423号