当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient access to multi-substituted 1-aminoisoquinolines via Rh(III)-catalyzed oxidative annulation of aminopyridine pivalamides with internal alkynes
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2022-06-25 , DOI: 10.1016/j.tetlet.2022.153970 Xiaoting Gan , Fen Huang , Zhijun Ren , Bo Li , Shaojie Chen , Wenkun Luo , Jun Zhou
中文翻译:

通过 Rh(III) 催化的氨基吡啶新戊酰胺与内部炔烃的氧化环化有效获得多取代的 1-氨基异喹啉
更新日期:2022-06-25
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2022-06-25 , DOI: 10.1016/j.tetlet.2022.153970 Xiaoting Gan , Fen Huang , Zhijun Ren , Bo Li , Shaojie Chen , Wenkun Luo , Jun Zhou
|
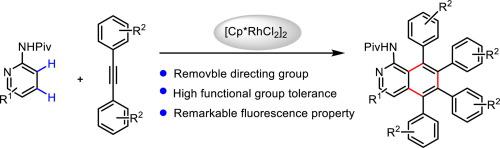
The synthesis of multi-substituted 1-aminoisoquinolines from aminopyridine pivalamides with alkynes by Rh(III)-catalyzed C H activation coupling has been investigated. This method proved to be tolerant of synthetically versatile functional groups, and provided access to a few highly substituted isoquinoline derivatives with remarkable fluorescence properties in moderate yields.
H activation coupling has been investigated. This method proved to be tolerant of synthetically versatile functional groups, and provided access to a few highly substituted isoquinoline derivatives with remarkable fluorescence properties in moderate yields.
中文翻译:

通过 Rh(III) 催化的氨基吡啶新戊酰胺与内部炔烃的氧化环化有效获得多取代的 1-氨基异喹啉
 研究了由氨基吡啶新戊酰胺与炔烃通过 Rh(III) 催化的 C H 活化偶联合成多取代的 1-氨基异喹啉。该方法被证明可以耐受合成的多功能官能团,并以中等产率提供了一些具有显着荧光特性的高度取代的异喹啉衍生物。
研究了由氨基吡啶新戊酰胺与炔烃通过 Rh(III) 催化的 C H 活化偶联合成多取代的 1-氨基异喹啉。该方法被证明可以耐受合成的多功能官能团,并以中等产率提供了一些具有显着荧光特性的高度取代的异喹啉衍生物。































 京公网安备 11010802027423号
京公网安备 11010802027423号