Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2022-06-24 , DOI: 10.1016/j.freeradbiomed.2022.06.241 Xiaomeng Zhang 1 , Dezhen Tu 2 , Sheng Li 3 , Na Li 3 , Donglai Li 3 , Yun Gao 2 , Lu Tian 3 , Jianing Liu 3 , Xuan Zhang 3 , Jau-Shyong Hong 2 , Liyan Hou 3 , Jie Zhao 3 , Qingshan Wang 1
|
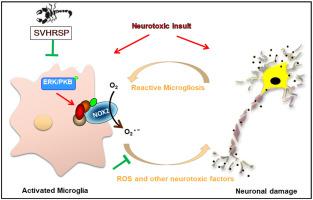
Current treatment of Parkinson's disease (PD) ameliorates symptoms but fails to block disease progression. This study was conducted to explore the protective effects of SVHRSP, a synthetic heat-resistant peptide derived from scorpion venom, against dopaminergic neurodegeneration in experimental models of PD. Results showed that SVHRSP dose-dependently reduced the loss of dopaminergic neuron in the nigrostriatal pathway and motor impairments in both rotenone and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/probenecid (MPTP/p)-induced mouse PD models. Microglial activation and imbalance of M1/M2 polarization were also abrogated by SVHRSP in both models. In rotenone-treated primary midbrain neuron-glial cultures, loss of dopaminergic neuron and microglial activation were mitigated by SVHRSP. Furthermore, lipopolysaccharide (LPS)-elicited microglial activation, M1 polarization and related dopaminergic neurodegeneration in primary cultures were also abrogated by SVHRSP, suggesting that inhibition of microglial activation contributed to SVHRSP-afforded neuroprotection. Mechanistic studies revealed that SVHRSP blocked both LPS- and rotenone-induced microglial NADPH oxidase (NOX2) activation by preventing membrane translocation of cytosolic subunit p47phox. NOX2 knockdown by siRNA markedly attenuated the inhibitory effects of SVHRSP against LPS- and rotenone-induced gene expressions of proinflammatory factors and related neurotoxicity. Altogether, SVHRSP protects dopaminergic neurons by blocking NOX2-mediated microglial activation in experimental PD models, providing experimental basis for the screening of clinical therapeutic drugs for PD.
中文翻译:

一种新型合成肽 SVHRSP 通过抑制帕金森病实验模型中 NADPH 氧化酶介导的神经炎症来减轻多巴胺能神经变性
目前对帕金森病 (PD) 的治疗可以改善症状,但不能阻止疾病进展。本研究旨在探讨 SVHRSP(一种源自蝎毒的合成耐热肽)在 PD 实验模型中对多巴胺能神经退行性变的保护作用。结果表明,SVHRSP 剂量依赖性地减少黑质纹状体通路中多巴胺能神经元的损失以及鱼藤酮和 1-甲基-4-苯基-1,2,3,6-四氢吡啶/丙磺舒 (MPTP/p) 诱导的运动损伤小鼠 PD 模型。在两种模型中,SVHRSP 也消除了小胶质细胞激活和 M1/M2 极化的不平衡。在鱼藤酮处理的原代中脑神经元胶质培养物中,SVHRSP 减轻了多巴胺能神经元的损失和小胶质细胞的活化。此外,SVHRSP 也消除了原代培养物中脂多糖 (LPS) 引发的小胶质细胞活化、M1 极化和相关的多巴胺能神经变性,这表明抑制小胶质细胞活化有助于 SVHRSP 提供的神经保护。机制研究表明,SVHRSP 通过阻止细胞溶质亚基 p47phox 的膜易位来阻断 LPS 和鱼藤酮诱导的小胶质细胞 NADPH 氧化酶 (NOX2) 活化。siRNA 的 NOX2 敲低显着减弱了 SVHRSP 对 LPS 和鱼藤酮诱导的促炎因子基因表达和相关神经毒性的抑制作用。总之,SVHRSP在实验性PD模型中通过阻断NOX2介导的小胶质细胞活化来保护多巴胺能神经元,为PD临床治疗药物的筛选提供实验依据。































 京公网安备 11010802027423号
京公网安备 11010802027423号