当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
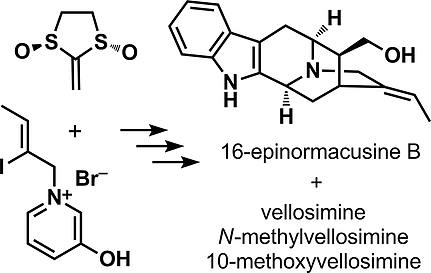
Total Syntheses of Vellosimine,N-Methylvellosimine, and 10-Methoxyvellosimine and Formal Synthesis of 16-Epinormacusine B through a [5+2] Cycloaddition
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2016-10-01 , DOI: 10.1002/ejoc.201600870 Sebastian Krüger 1 , Tanja Gaich 2
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2016-10-01 , DOI: 10.1002/ejoc.201600870 Sebastian Krüger 1 , Tanja Gaich 2
Affiliation
|
To date, more than 100 members of the sarpagine alkaloid family have been isolated. Their structural variations originate from oxidative transformations of the carboskeleton and the presence of both absolute configurations at the C-16 atom, which is established in the course of their biosynthesis. More than 40 sarpagine alkaloids belong to the either the 16-regular or 16-epi subgroups, depending on the stereochemistry at C-16. Herein, we report the formal synthesis of 16-epinormacusine B, a member of the 16-epi group, by using our well-established generalized strategy for the total synthesis of these alkaloids. Furthermore, we provide the synthetic details and pitfalls of the asymmetric total syntheses of vellosimine, N-methylvellosimine, and 10-methoxyvellosimine, all members of the 16-regular group.
中文翻译:

Vellosimine、N-Methylvelosimine 和 10-Methoxyvelosimine 的全合成以及 16-Epinormacusine B 通过 [5+2] 环加成的形式合成
迄今为止,已分离出超过 100 名沙巴金生物碱家族成员。它们的结构变化源于碳骨架的氧化转化和 C-16 原子上两种绝对构型的存在,这是在它们的生物合成过程中建立的。根据 C-16 的立体化学,超过 40 种 sarpagine 生物碱属于 16-regular 或 16-epi 亚组。在此,我们报告了 16-epi 组成员 16-epinormacusine B 的正式合成,使用我们完善的通用策略来全合成这些生物碱。此外,我们提供了 velosimine、N-methylvelosimine 和 10-methoxyvelosimine 的不对称全合成的合成细节和陷阱,这些都是 16-regular group 的成员。
更新日期:2016-10-01
中文翻译:

Vellosimine、N-Methylvelosimine 和 10-Methoxyvelosimine 的全合成以及 16-Epinormacusine B 通过 [5+2] 环加成的形式合成
迄今为止,已分离出超过 100 名沙巴金生物碱家族成员。它们的结构变化源于碳骨架的氧化转化和 C-16 原子上两种绝对构型的存在,这是在它们的生物合成过程中建立的。根据 C-16 的立体化学,超过 40 种 sarpagine 生物碱属于 16-regular 或 16-epi 亚组。在此,我们报告了 16-epi 组成员 16-epinormacusine B 的正式合成,使用我们完善的通用策略来全合成这些生物碱。此外,我们提供了 velosimine、N-methylvelosimine 和 10-methoxyvelosimine 的不对称全合成的合成细节和陷阱,这些都是 16-regular group 的成员。






























 京公网安备 11010802027423号
京公网安备 11010802027423号