Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2022-06-23 , DOI: 10.1016/j.molliq.2022.119691
Murielly Fernanda Ribeiro Bihain , Ellane Jacqueline Coelho Moreira Gomes , Vinicius Souza Macedo , Grasiele Soares Cavallini , Douglas Henrique Pereira
|
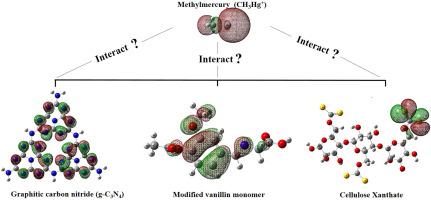
In this study, the adsorptive capacity of contaminant methylmercury (CH3Hg+) in the matrices of graphitic carbon nitride (g-C3N4), cellulose xanthate (XC), and modified vanillin monomer (VN) was theoretically determined using ωB97X-D/6-31 + G(d,p)/LANL2DZ level. The location of possible interaction sites between the structures was verified using an molecular eletrostatic potential, frontier molecular orbital, and atomic charges, which indicated that for the g-C3N4 matrix, the CH3Hg+ interacted preferentially at the center of the molecule. Conversely, for the XC and VN matrices, this interaction occurred in the -CS2- group and on the Schiff base, respectively. The reactivity indices indicated that the VN matrix demonstrates better interaction with CH3Hg+. Among the evaluated interaction sites, the Schiff base was the most effective for the interaction with methylmercury with a ΔEBind value of −4.80 kJ mol−1. Topological analyses showed that the XC-CH3Hg1 and XC-CH3Hg2 complexes and the interaction “b” for g-C3N4-CH3Hg2 interacted with a partially covalent character. The complexes g-C3N4-CH3Hg1, g-C3N4-CH3Hg3, VN-CH3Hg1, and the interaction “a” of the complex g-C3N4-CH3Hg2 exhibited electrostatic characteristics. Finally, based on the theoretical results, it can be affirmed that the Schiff base is the best adsorption site for CH3Hg+, evidencing that this study can support further experimental essays to remove contaminants from effluents.
中文翻译:

关于使用不同吸附基质去除 CH3Hg+ 可能性的理论见解:g-C3N4、黄原酸纤维素和香草醛衍生的改性单体
在这项研究中,使用 ωB97X-D 从理论上测定了石墨氮化碳 (gC 3 N 4 )、黄原酸纤维素 (XC) 和改性香草醛单体 (VN)基质中污染物甲基汞 (CH 3 Hg + ) 的吸附能力。 /6-31 + G(d,p)/LANL2DZ 级别。使用分子静电势、前沿分子轨道和原子电荷验证了结构之间可能的相互作用位点的位置,这表明对于gC 3 N 4基质,CH 3 Hg +优先在分子中心相互作用。相反,对于 XC 和 VN 矩阵,这种交互发生在 -CS 2-组和席夫基地,分别。反应性指数表明,VN 基质与 CH 3 Hg +有更好的相互作用。在评估的相互作用位点中,席夫碱对与甲基汞的相互作用最有效,ΔE Bind值为 -4.80 kJ mol -1。拓扑分析表明XC-CH 3 Hg 1和XC-CH 3 Hg 2配合物以及gC 3 N 4 -CH 3 Hg 2的相互作用“ b ”具有部分共价特征。配合物 gC 3N 4 -CH 3 Hg 1、gC 3 N 4 -CH 3 Hg 3、VN-CH 3 Hg 1和配合物gC 3 N 4 -CH 3 Hg 2的相互作用“ a ”表现出静电特性。最后,基于理论结果,可以肯定席夫碱是CH 3 Hg +的最佳吸附位点,证明本研究可以支持进一步的实验论文以去除废水中的污染物。

































 京公网安备 11010802027423号
京公网安备 11010802027423号