Bioactive Materials ( IF 18.0 ) Pub Date : 2022-06-21 , DOI: 10.1016/j.bioactmat.2022.06.010 Yue Xu 1 , Yuan You 1 , Luyao Yi 1 , Xiaoyi Wu 1 , Yaning Zhao 1 , Jian Yu 1 , He Liu 2 , Ya Shen 2 , Jingmei Guo 1 , Cui Huang 1
|
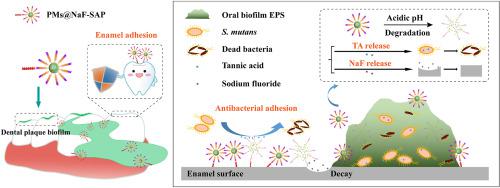
Dental caries is one of the most prevalent human diseases resulting from tooth demineralization caused by acid production of bacteria plaque. It remains challenges for current practice to specifically identify, intervene and interrupt the development of caries while restoring defects. In this study, inspired by natural dental plaque, a stimuli-responsive multidrug delivery system (PMs@NaF-SAP) has been developed to prevent tooth decay and promote enamel restoration. Classic spherical core-shell structures of micelles dual-loaded with antibacterial and restorative agents are self-assembled into bacteria-responsive multidrug delivery system based on the pH-cleavable boronate ester bond, followed by conjugation with salivary-acquired peptide (SAP) to endow the nanoparticle with strong adhesion to tooth enamel. The constructed PMs@NaF-SAP specifically adheres to tooth, identifies cariogenic conditions and intelligently releases drugs at acidic pH, thereby providing antibacterial adhesion and cariogenic biofilm resistance, and restoring the microarchitecture and mechanical properties of demineralized teeth. Topical treatment with PMs@NaF-SAP effectively diminishes the onset and severity of caries without impacting oral microbiota diversity or surrounding mucosal tissues. These findings demonstrate this novel nanotherapy has potential as a promising biomedical application for caries prevention and tooth defect restoration while resisting biofilm-associated diseases in a controlled manner activated by pathological bacteria.
中文翻译:

牙菌斑启发的多功能纳米系统用于龋齿预防和牙齿修复
龋齿是最普遍的人类疾病之一,由细菌菌斑产生酸引起的牙齿脱矿质引起。在修复缺陷的同时,具体识别、干预和中断龋齿的发展仍然是当前实践的挑战。在这项研究中,受天然牙菌斑的启发,开发了一种刺激响应的多药递送系统 (PMs@NaF-SAP),以防止蛀牙和促进牙釉质修复。经典的球状核壳结构胶束双载抗菌和修复剂,基于 pH 可裂解的硼酸酯键自组装成细菌响应的多药递送系统,然后与唾液获得性肽 (SAP) 缀合以赋予纳米粒子对牙釉质有很强的附着力。所构建的 PMs@NaF-SAP 特异性粘附于牙齿,识别致龋情况并在酸性 pH 下智能释放药物,从而提供抗菌粘附和致龋生物膜抗性,并恢复脱矿牙齿的微结构和机械性能。PMs@NaF-SAP 的局部治疗有效地减少了龋齿的发生和严重程度,而不影响口腔微生物群的多样性或周围的粘膜组织。这些发现表明,这种新型纳米疗法具有作为预防龋齿和牙齿缺损修复的有前景的生物医学应用潜力,同时以受病态细菌激活的受控方式抵抗生物膜相关疾病。从而提供抗菌粘附和致龋生物膜抗性,并恢复脱矿质牙齿的微结构和机械性能。PMs@NaF-SAP 的局部治疗有效地减少了龋齿的发生和严重程度,而不影响口腔微生物群的多样性或周围的粘膜组织。这些发现表明,这种新型纳米疗法具有作为预防龋齿和牙齿缺损修复的有前景的生物医学应用潜力,同时以受病态细菌激活的受控方式抵抗生物膜相关疾病。从而提供抗菌粘附和致龋生物膜抗性,并恢复脱矿质牙齿的微结构和机械性能。PMs@NaF-SAP 的局部治疗有效地减少了龋齿的发生和严重程度,而不影响口腔微生物群的多样性或周围的粘膜组织。这些发现表明,这种新型纳米疗法具有作为预防龋齿和牙齿缺损修复的有前景的生物医学应用潜力,同时以受病态细菌激活的受控方式抵抗生物膜相关疾病。PMs@NaF-SAP 的局部治疗有效地减少了龋齿的发生和严重程度,而不影响口腔微生物群的多样性或周围的粘膜组织。这些发现表明,这种新型纳米疗法具有作为预防龋齿和牙齿缺损修复的有前景的生物医学应用潜力,同时以受病态细菌激活的受控方式抵抗生物膜相关疾病。PMs@NaF-SAP 的局部治疗有效地减少了龋齿的发生和严重程度,而不影响口腔微生物群的多样性或周围的粘膜组织。这些发现表明,这种新型纳米疗法具有作为预防龋齿和牙齿缺损修复的有前景的生物医学应用潜力,同时以受病态细菌激活的受控方式抵抗生物膜相关疾病。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号