Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2022-06-21 , DOI: 10.1016/j.apcatb.2022.121652 Zhanghao Chen , Ying Teng , Wenran Wang , Ran Hong , Liuqing Huang , Xinhao Wang , Fengxiao Zhu , Hui Li , Shefeng Hao , Bing Wu , Cheng Gu
|
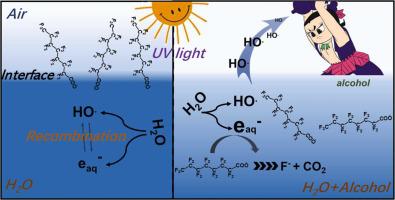
Herein, we developed a novel hydrated electron induced approach by simply adding alcohols (0.2 %~0.5 %) as catalysts to enhance the decomposition of perfluorooctanoic acid (PFOA). Different from previous studies, in which high concentration of hydrated electron source materials are generally required, the hydrated electron in this study was generated from UV photolysis of water molecules. Our results clearly demonstrated that the addition of alcohols could not only protect hydrated electron from quenching by hydroxyl radical, but also improve the dispersion of PFOA in solution, thus resulting in higher hydrated electron yield and utilization efficiency. Furthermore, owing to the quenching of proton and oxygen by the generated alcohol radical, the UV/H2O/alcohol system exhibited adaptability to different environmental variables, i.e., solution pH and reaction atmosphere. Therefore, the newly developed UV/H2O/alcohol system shows promising potential for treatment of PFOA-containing wastewater and investigator derived waste, as alcohol is usually used as the desorption agent.
中文翻译:

在醇存在下增强全氟辛酸的紫外光还原破坏:羟基自由基猝灭和溶剂效应的协同机制
在此,我们开发了一种新的水合电子诱导方法,通过简单地添加醇(0.2%~0.5%)作为催化剂来增强全氟辛酸(PFOA)的分解。与以往研究中通常需要高浓度的水合电子源材料不同,本研究中的水合电子是由水分子的紫外光解产生的。我们的研究结果清楚地表明,添加醇不仅可以保护水合电子不被羟基自由基猝灭,而且可以改善 PFOA 在溶液中的分散性,从而提高水合电子产率和利用效率。此外,由于生成的醇自由基对质子和氧的淬灭,UV/H 2O/醇体系表现出对不同环境变量的适应性,即溶液pH值和反应气氛。因此,新开发的 UV/H 2 O/酒精系统在处理含 PFOA 的废水和研究人员产生的废物方面显示出巨大的潜力,因为酒精通常用作解吸剂。

































 京公网安备 11010802027423号
京公网安备 11010802027423号